��Ŀ����
�յ�����������������������������Ӱ��յ�����������ͭ-���Ͻ����������������ijɹ����������������ҵ��һ�����⣬����Ϊ��ʮһ����������ҵ�����ԵĴ��£�
��1��Ϊ�ⶨ��ͭ-���Ͻ��������������5.6�˺Ͻ���Ʒ���飬Ͷ�뵽���������У���ַ�Ӧ��õ�����6.72L����״��������ͭ-���Ͻ���������������Ϊ ����ȷ��0.01����
��2����������ӦҺ���ˣ�������Һϡ�͵�100mL��д����Ҫ������̣�
��ϡ�ͺ���Һ��Al3+�����ʵ���Ũ��Ϊ���٣�
�ڴ�������Һ��ȡ��10mL��Ҫ����0.78�˳��������ټ���0.5mol/L NaOH��Һ�����Ϊ����mL��
��1��Ϊ�ⶨ��ͭ-���Ͻ��������������5.6�˺Ͻ���Ʒ���飬Ͷ�뵽���������У���ַ�Ӧ��õ�����6.72L����״��������ͭ-���Ͻ���������������Ϊ
��2����������ӦҺ���ˣ�������Һϡ�͵�100mL��д����Ҫ������̣�
��ϡ�ͺ���Һ��Al3+�����ʵ���Ũ��Ϊ���٣�
�ڴ�������Һ��ȡ��10mL��Ҫ����0.78�˳��������ټ���0.5mol/L NaOH��Һ�����Ϊ����mL��
���㣺�йػ���ﷴӦ�ļ���,��ѧ����ʽ���йؼ���
ר�⣺������
��������1��ͭ�������Ӧ���������ᷢ��2Al+6H+=2Al3++3H2����n��H2��=
=0.3mol�����ݷ���ʽ��������������������������������
��2������c=
����Ũ�ȣ������������ƣ��ɷ���Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O����Ϸ���ʽ���㣮
| 6.72L |
| 22.4L/mol |
��2������c=
| n |
| V |
���
�⣺��1��ͭ�������Ӧ���������ᷢ��2Al+6H+=2Al3++3H2����n��H2��=
=0.3mol��
�ɷ���ʽ��֪n��Al��=0.2mol��m��Al��=0.2mol��27g/mol=5.4g��
�أ�Al��=
=0.96��
�ʴ�Ϊ��0.96��
��2����������ӦҺ���ˣ�������Һϡ�͵�100mL��
��ϡ�ͺ���Һ��Al3+�����ʵ���Ũ��Ϊ
=2mol/L��
��ϡ�ͺ���Һ��Al3+�����ʵ���Ũ��Ϊ2mol/L��
�ڴ�������Һ��ȡ��10mL��n��Al3+��=2mol/L��0.01L=0.02mol��
n��Al��OH��3��=
=0.01mol��
��NaOH����ʱ��
Al3++3OH-=Al��OH��3��
3mol 1mol
x 0.01mol
��x=0.03mol��
V1��NaOH��Һ��=
=0.06L=60mL��
��ʹAl��OH��3�����ܽ⣬��
��Al3++3OH-=Al��OH��3��
0.02mol 0.06mol
��Al��OH��3+OH-=AlO2-+2H2O
1mol 1mol
��0.02-0.01��mol ��0.02-0.01��mol=0.01mol
������Ӧ����Ҫ0.06mol+0.01mol=0.07mol��
V2��NaOH��Һ��=
=0.14L=140mL��
�𣺴�������Һ��ȡ��10mL��Ҫ����0.78�˳��������ټ���0.5mol/L NaOH��Һ�����Ϊ60mL��140mL��
| 6.72L |
| 22.4L/mol |
�ɷ���ʽ��֪n��Al��=0.2mol��m��Al��=0.2mol��27g/mol=5.4g��
�أ�Al��=
| 5.4g |
| 5.6g |
�ʴ�Ϊ��0.96��
��2����������ӦҺ���ˣ�������Һϡ�͵�100mL��
��ϡ�ͺ���Һ��Al3+�����ʵ���Ũ��Ϊ
| 0.2mol |
| 0.1L |
��ϡ�ͺ���Һ��Al3+�����ʵ���Ũ��Ϊ2mol/L��
�ڴ�������Һ��ȡ��10mL��n��Al3+��=2mol/L��0.01L=0.02mol��
n��Al��OH��3��=
| 0.78g |
| 78g/mol |
��NaOH����ʱ��
Al3++3OH-=Al��OH��3��
3mol 1mol
x 0.01mol
��x=0.03mol��
V1��NaOH��Һ��=
| 0.03mol |
| 0.5mol/L |
��ʹAl��OH��3�����ܽ⣬��
��Al3++3OH-=Al��OH��3��
0.02mol 0.06mol
��Al��OH��3+OH-=AlO2-+2H2O
1mol 1mol
��0.02-0.01��mol ��0.02-0.01��mol=0.01mol
������Ӧ����Ҫ0.06mol+0.01mol=0.07mol��
V2��NaOH��Һ��=
| 0.07mol |
| 0.5mol/L |
�𣺴�������Һ��ȡ��10mL��Ҫ����0.78�˳��������ټ���0.5mol/L NaOH��Һ�����Ϊ60mL��140mL��
���������⿼�黯ѧ����ʽ����ؼ��㣬������ѧ���ķ��������ͼ��������Ŀ��飬��Ŀ�Ѷ��еȣ�ע���ж���������Ӧ�ij̶ȣ�Ϊ�����Ĺؼ��������۷����

��ϰ��ϵ�д�
�����Ŀ
��ɫ�Ļ������ף����ܺ�NO��CO2��NO2��N2�еļ��֣���һ�����ļ����徭����ͼʵ��Ĵ���������õ�������Һ����������ʣ�࣬�����������Ϊ��������
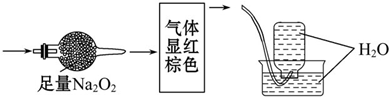

| A��NO2��N2 |
| B��NO��CO2 |
| C��NO2��CO2 |
| D��NO��CO2��N2 |
��б������������������ͬ�������壮
��֪����S��s����б��+O2��g��=SO2��g����H1=-297.16kJ?mol-1
��S��s��������+O2��g��=SO2 ��g����H2=-296.83kJ?mol -1
����˵����ȷ���ǣ�������
��֪����S��s����б��+O2��g��=SO2��g����H1=-297.16kJ?mol-1
��S��s��������+O2��g��=SO2 ��g����H2=-296.83kJ?mol -1
����˵����ȷ���ǣ�������
| A��S��s�����=S��s����������H3=+0.33kJ?mol -1 |
| B����б����������ȶ� |
| C������ʽ���κ��¶��¶����Է����У�˵����ʽ���ر��S��0 |
| D����ʽ��ʾ����lmol O2�еĹ��ۼ������յ��������γ�1mol SO2�еĹ��ۼ����ų���������297.16kJ |
 һ���¶���2L�ĺ����������У�����2moL̼��2moLCO2�������·�Ӧ��
һ���¶���2L�ĺ����������У�����2moL̼��2moLCO2�������·�Ӧ��
