��Ŀ����
13����֪5�ֶ�����Ԫ�ص�ԭ��������E��D��B��A��C��˳����������A��Cͬ���ڣ�B��Cͬ���壻A��B�γ����ӻ�����A2B��A2B���������ӵĵ�������ͬ��A2B�е�������Ϊ30��D��E���γ�4��10���ӵķ��ӣ��Իش��������⣺��1��д��5��Ԫ�ص����ƣ�A�ơ�B����C��D����E�⣮
��2��д���������ʵĵ���ʽ��DԪ���γɵĵ���
 ��B��E�γɵĻ�����
��B��E�γɵĻ����� ��A��B��E�γɵĻ�����Na+
��A��B��E�γɵĻ�����Na+ ��D��E�γɵĻ�����
��D��E�γɵĻ����� ��
����3��A��B��Ԫ����ɵĻ�����A2B2�������ӣ�����ӡ����ۡ�����������ڵĻ�ѧ�������Ӽ������ۼ���д��A2B2��ˮ��Ӧ�Ļ�ѧ����ʽ2Na2O2+2H2O=4NaOH+O2����
���� 5�ֶ�����Ԫ�ص�ԭ��������E��D��B��A��C��˳����������D��E���γ�4��10���ӵķ��ӣ������Ϊ��������EΪH��DΪN��A��B���γ����ӻ�����A2B��A2B���������ӵĵ�������ͬ���ҵ�������Ϊ30����AΪNa��BΪO��A��Cͬ���ڣ�B��Cͬ���壬��֪CΪS���Դ������
��� �⣺��1�������Ϸ�����֪AΪ��Ԫ�أ�BΪ����CΪ��DΪ����EΪ��Ԫ�أ�
�ʴ�Ϊ���ƣ����������⣻
��2��DԪ���γɵĵ���N2�к��е�������������ʽ���Ա�ʾΪ ����Ԫ�غ���Ԫ���γɵĻ�����ˮ������Ԫ�غ���Ԫ��֮��ͨ�����ۼ��γɵģ�����ʽΪ��
����Ԫ�غ���Ԫ���γɵĻ�����ˮ������Ԫ�غ���Ԫ��֮��ͨ�����ۼ��γɵģ�����ʽΪ�� ��Na��O��H�γɵĻ�������NaOH������ʽΪNa+
��Na��O��H�γɵĻ�������NaOH������ʽΪNa+ ��H��N�γɵĻ�������NH3������ʽΪ
��H��N�γɵĻ�������NH3������ʽΪ ��
��
�ʴ�Ϊ�� ��
�� ��Na+
��Na+ ��
�� ��
��
��3��Na2O2Ϊ���ӻ����������Ӽ����ۼ�����ˮ��Ӧ�����������ƺ���������Ӧ�ķ���ʽΪ2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ�����ӣ����Ӽ������ۼ���2Na2O2+2H2O=4NaOH+O2����
���� ���⿼��ṹ����λ�ù�ϵ��Ϊ��Ƶ���㣬�漰���û�ѧ������ƶϡ�Ԫ�ػ�����֪ʶ�ȣ����ط����ƶ��������ۺϿ��飬��Ŀ�Ѷ��е�

��ϰ��ϵ�д�
�����Ŀ
19��һ�������̲�Ӧ����������ɣ��ǡ��̡��裮�̾���ԭ�����⻯ֲ���ͣ����̲�۷ŵö࣬�����̲�Ŀڸоͺã��ɱ�Ҳ�ߣ�������ͳɱ��������������Ӱ��ǻ������㾫���̷ۣ�����˵����ȷ���ǣ�������
| A�� | �̾���ʹ��ˮ������KMnO4��Һ��ɫ����ԭ����ͬ | |
| B�� | �⻯ֲ������ϡH2SO4��NaOH��Һ�з���ˮ�⣬���ò�����ͬ | |
| C�� | ��ɰ����������ˮ��ת��Ϊ���Ƕ����������� | |
| D�� | �̷ۿ���Ϊ���������һ��Ӫ������ |
1����֪25�桢101kPa �£�ʯī�����ʯȼ�յ��Ȼ�ѧ����ʽ���£�C��ʯī��+O2��g��=CO2��g������H=-393.51kJ•mol-1�� C�����ʯ��+O2��g��=CO2��g������H=-395.41kJ•mol-1������˵����ȷ���ǣ�������
| A�� | ���ʯ��ʯī�ȶ� | B�� | ʯīת��Ϊ���ʯ��Ҫ���� | ||
| C�� | ���ʯȼ�ղ�����ȶ� | D�� | ������ʱ��ʯī���������� |
8�������й�˵��������ǣ�������
| A�� | �ѻ����Ͳ���������ȡ��ˮ�е��� | |
| B�� | ����ʽΪC5H10O2�����뱥��NaHCO3��Һ��Ӧ�ų�������л����У����������칹��4�� | |
| C�� | ʵ�����У����ý����Ƽ����Ҵ����Ƿ���ˮ | |
| D�� | ��֬�ڼ��������µ�ˮ�ⷴӦ��Ϊ������Ӧ |
18�������л���Ӧ����ʽ�У�����ȷ���ǣ�������
| A�� | ��ϩ��Br2�ӳɣ�CH2�TCHCH3+Br2��CH2BrCH2CH2Br | |
| B�� | ������Cl2����ȡ����Ӧ��CH4+Cl2$\stackrel{����}{��}$CH3Cl+HCl | |
| C�� | ���ѻ���C16H34$��_{��}^{����}$C8H16+C8H18 | |
| D�� | ����ˮ�⣺��C6H10O5��n+nH2O$\stackrel{����}{��}$nC6H12O6 |
5��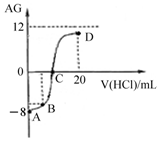 ����AG��ʾ��Һ����ȣ������ʽΪ��AG=lg[$\frac{{c��{H^+}��}}{{c��O{H^-}��}}$]�������£�ʵ��������0.1mol/L��������Һ�ζ�10mL 0.1mol/L MOH��Һ���ζ�������ͼ��ʾ������˵����ȷ���ǣ�������
����AG��ʾ��Һ����ȣ������ʽΪ��AG=lg[$\frac{{c��{H^+}��}}{{c��O{H^-}��}}$]�������£�ʵ��������0.1mol/L��������Һ�ζ�10mL 0.1mol/L MOH��Һ���ζ�������ͼ��ʾ������˵����ȷ���ǣ�������
 ����AG��ʾ��Һ����ȣ������ʽΪ��AG=lg[$\frac{{c��{H^+}��}}{{c��O{H^-}��}}$]�������£�ʵ��������0.1mol/L��������Һ�ζ�10mL 0.1mol/L MOH��Һ���ζ�������ͼ��ʾ������˵����ȷ���ǣ�������
����AG��ʾ��Һ����ȣ������ʽΪ��AG=lg[$\frac{{c��{H^+}��}}{{c��O{H^-}��}}$]�������£�ʵ��������0.1mol/L��������Һ�ζ�10mL 0.1mol/L MOH��Һ���ζ�������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �õζ����̿�ѡ���̪��Ϊָʾ�� | |
| B�� | ��B������������Һ���Ϊ5 mL��������Һ�У�c��M+��+2c��H+��=c��MOH��+2c��OH-�� | |
| C�� | �ζ������д�A�㵽D����Һ��ˮ�ĵ���̶ȣ�A��B��C��D | |
| D�� | C��ʱ����������Һ���������10 mL |
2�����й��ڻ�ѧ�������˵������ȷ���ǣ�������
| A�� | �����Ҵ��Ļ�ԭ���Լ�Cr3+��Cr2O72-����ɫ����������ƺ�ݳ� | |
| B�� | �ߴ�����Խ�̫����ֱ��ת��Ϊ���� | |
| C�� | ��ѧҩƷ�Ż𣬶�Ҫ������ˮ����ĭ�������� | |
| D�� | ��ҵ��Cl2����ʯ���鷴Ӧ�Ʊ�Ư�� |
3���������м����ͨ�������������ʣ���Һ������ǿ���ǣ�������
| A�� | O2 | B�� | Cl2 | C�� | SO2 | D�� | NaOH |



