��Ŀ����
�����ʵ���A��B�����2L���ܱ������У��������·�Ӧ��3A��g��+B��g��?xC��g��+2D��g����Լ5min���D��Ũ��Ϊ0.5mol/L��c��A����c��B��=3��5��C��ƽ����Ӧ����Ϊ0.05mol/��L?min������
��1��5minʱA��Ũ��c��A������Ӧ��ʼǰ�����е�A��B�����ʵ�����
��2��ǰ5min����B��ʾ��ƽ����Ӧ���ʣ�
��3����ѧ��Ӧ����ʽ��x��ֵ��
��4��5minʱ����A��ת���ʣ�
��5�����÷�Ӧһ�����������Է���Ӧ����÷�Ӧ�ġ�H ���������������=����0��
��1��5minʱA��Ũ��c��A������Ӧ��ʼǰ�����е�A��B�����ʵ�����
��2��ǰ5min����B��ʾ��ƽ����Ӧ���ʣ�
��3����ѧ��Ӧ����ʽ��x��ֵ��
��4��5minʱ����A��ת���ʣ�
��5�����÷�Ӧһ�����������Է���Ӧ����÷�Ӧ�ġ�H
���㣺��ѧƽ��ļ���
ר�⣺��ѧƽ��ר��
��������1������D��Ũ�����D�����ʵ�������Ϸ���ʽ����ʽ���5minʱA��Ũ��c��A����A��B�����ʵ�����
��2���������Ӧ��B�����ʵ������ٸ���ƽ����Ӧ���ʹ�ʽ����B��ƽ����Ӧ���ʣ�
��3������ͬһ��Ӧ�з�Ӧ����֮�ȵ��ڻ�ѧ������֮�����xֵ��
��4������ת����=
��100%���м��㣻
��5�����ݡ�G=��H-T��S�����жϣ�
��2���������Ӧ��B�����ʵ������ٸ���ƽ����Ӧ���ʹ�ʽ����B��ƽ����Ӧ���ʣ�
��3������ͬһ��Ӧ�з�Ӧ����֮�ȵ��ڻ�ѧ������֮�����xֵ��
��4������ת����=
| ������ |
| ��ʼ�� |
��5�����ݡ�G=��H-T��S�����жϣ�
���
�⣺��1��5���Ӻ�n��D��=CV=0.5mol/L��2L=1mol���跴Ӧ��ʼǰ����������A��B���ʵ���Ϊmmol��
3A��g��+B��g��?xC��g��+2D��g����
��Ӧǰ m mol m mol 0 0
5���Ӻ� �� m-1.5��mol �� m-0.5��mol 1mol
c��A����c��B��=3��5=�� m-1.5��mol���� m-0.5��mol
m=3 mol��
5minʱA��Ũ��c��A��=
=0.75mol/L��
��5minʱA��Ũ��c��A��Ϊ0.75mol/L����Ӧ��ʼǰ����������A��B���ʵ�����Ϊ3mol��
��2���跴Ӧ��B�����ʵ���Ϊnmol��
3A��g��+B��g��?xC��g��+2D��g����
1 2
nmol 1mol
n=0.5
����v��B��=
=0.05 mol/��L��min��
��B��ƽ����Ӧ����Ϊ0.05 mol/��L��min����
��3������ͬһ��Ӧ�з�Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ�����v��B����v��C��=0.05 mol/��L��min����0.05mol/��L?min��=1��x������x=1��
��xֵ��1��
��4�����ݣ�1���м������ݣ�5minʱ����A��ת����=
��100%=50%��
��5minʱ����A��ת����Ϊ50%��
��5������3A��g��+B��g��?C��g��+2D��g������Ӧ�����������С���S��0����ϸ÷�Ӧһ�����������Է���Ӧ�����G=��H-T��S��һ��������С��0�����ԡ�H��0��
�ʴ𰸣�����
3A��g��+B��g��?xC��g��+2D��g����
��Ӧǰ m mol m mol 0 0
5���Ӻ� �� m-1.5��mol �� m-0.5��mol 1mol
c��A����c��B��=3��5=�� m-1.5��mol���� m-0.5��mol
m=3 mol��
5minʱA��Ũ��c��A��=
| 1.5mol |
| 2L |
��5minʱA��Ũ��c��A��Ϊ0.75mol/L����Ӧ��ʼǰ����������A��B���ʵ�����Ϊ3mol��
��2���跴Ӧ��B�����ʵ���Ϊnmol��
3A��g��+B��g��?xC��g��+2D��g����
1 2
nmol 1mol
n=0.5
����v��B��=
| ��n |
| V����t |
��B��ƽ����Ӧ����Ϊ0.05 mol/��L��min����
��3������ͬһ��Ӧ�з�Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ�����v��B����v��C��=0.05 mol/��L��min����0.05mol/��L?min��=1��x������x=1��
��xֵ��1��
��4�����ݣ�1���м������ݣ�5minʱ����A��ת����=
| 1.5 |
| 3 |
��5minʱ����A��ת����Ϊ50%��
��5������3A��g��+B��g��?C��g��+2D��g������Ӧ�����������С���S��0����ϸ÷�Ӧһ�����������Է���Ӧ�����G=��H-T��S��һ��������С��0�����ԡ�H��0��
�ʴ𰸣�����
���������⿼������������ʽ���Ի�ѧƽ��ļ����Լ���Ӧ�Է����Է����жϣ���ȷͬһ��Ӧ�з�Ӧ����֮�ȵ��ڻ�ѧ������֮���ǽ⣨3���Ĺؼ���

��ϰ��ϵ�д�
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
�����Ŀ
��������Һ��BaSO4�ܽ������ǵģ�������
| A��1mol/L H2SO4��Һ |
| B��0.5mol/L BaCl2��Һ |
| C��0.1mol/L Na2SO4��Һ |
| D��0.2mol/L ��NH4��2SO4��Һ |
�����Ʊ������ʵ�鷽����ȷ���ǣ�������
| A����CO2 ����ʯ+ϡH2SO4 |
| B����Cl2 KMnO4����+Ũ���� |
| C����HCl ����ʳ��ˮ+ŨH2SO4 |
| D����C2H4 �Ҵ�+ŨH2SO4������140�� |

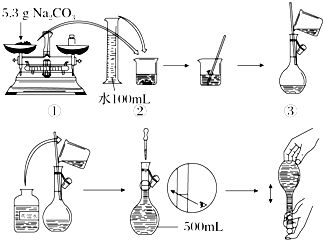 ��Na2CO3?10H2O���壬����0.2mol?L-1��Na2CO3��Һ480mL��
��Na2CO3?10H2O���壬����0.2mol?L-1��Na2CO3��Һ480mL��