��Ŀ����
 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һ������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һ������д���пհף���1��ѡ�õ�ָʾ����
��2���ñ���������Һ�ζ����������������Һʱ�����ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��
��3�����в����п���ʹ��������������Һ��Ũ����ֵƫ�͵���
A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
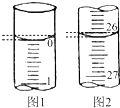
��4�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ���뽫���������������Ŀհ״���
| �ζ����� | ��������������Һ�����/mL | 0.1000mol/L ��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | |||
| �ڶ��� | 25.00 | 2.00 | 28.08 | 26.08 |
| ������ | 25.00 | 0.22 | 26.34 | 26.12 |
��6���ζ��յ���ж�������
���㣺�к͵ζ�
ר�⣺
��������1�������������������ǡ�÷�Ӧ��Һ�����ԣ���ѡ����Ա�ɫ��Χ�ڵ�ָʾ����̪��
��2������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯��
��3������c�����⣩=
����������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��4�����ݵζ��ܵĽṹ�;�ȷ���Լ�������ԭ����
��5���ȸ������ݵ���Ч�����ƽ������V�����ᣩ�����Ÿ��ݷ�Ӧ��HCl+NaOH=NaCl+H2O���C��NaOH����
��6��ѡ���̪��ָʾ�����ζ��յ�ʱ��Һ��ɫ��dz��ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
��2������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯��
��3������c�����⣩=
| c(��)��V(��) |
| V(����) |
��4�����ݵζ��ܵĽṹ�;�ȷ���Լ�������ԭ����
��5���ȸ������ݵ���Ч�����ƽ������V�����ᣩ�����Ÿ��ݷ�Ӧ��HCl+NaOH=NaCl+H2O���C��NaOH����
��6��ѡ���̪��ָʾ�����ζ��յ�ʱ��Һ��ɫ��dz��ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
���
�⣺��1���������������ǡ�÷�Ӧ��Һ�����ԣ���ѡ����Ա�ɫ��Χ�ڵ�ָʾ����̪���ʴ�Ϊ����̪��
��2������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯�����ж��յ㣬�ʴ�Ϊ����ƿ����Һ����ɫ�仯��
��3��A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=
����֪c�����⣩ƫ��A����
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬���V���������䣬����c�����⣩=
����֪c�����⣩���䣬��B����
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ����c�����⣩=
����֪c�����⣩ƫ��C����
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС������c�����⣩=
����֪c�����⣩ƫС����D��ȷ��
��ѡ��D��
��4����ʼ����Ϊ0.00mL���յ����Ϊ26.10mL��������Һ�����Ϊ26.10mL��
�ʴ�Ϊ��0.00��26.10��26.10��
��5���������ݾ���Ч��ƽ������V�����ᣩ=
mL=26.10mL��
HCl+NaOH=NaCl+H2O
0.0261L��0.1000mol/L 0.025L��C��NaOH��
��C��NaOH��=
=0.1044mol/L��
�ʴ�Ϊ��0.1044mol/L��
��6��ѡ���̪��ָʾ�����ζ��յ�ʱ��Һ��ɫ��dz��ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ���ʴ�Ϊ����Һ��ɫ��dz��ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
��2������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯�����ж��յ㣬�ʴ�Ϊ����ƿ����Һ����ɫ�仯��
��3��A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=
| c(��)��V(��) |
| V(����) |
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬���V���������䣬����c�����⣩=
| c(��)��V(��) |
| V(����) |
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ����c�����⣩=
| c(��)��V(��) |
| V(����) |
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС������c�����⣩=
| c(��)��V(��) |
| V(����) |
��ѡ��D��
��4����ʼ����Ϊ0.00mL���յ����Ϊ26.10mL��������Һ�����Ϊ26.10mL��
�ʴ�Ϊ��0.00��26.10��26.10��
��5���������ݾ���Ч��ƽ������V�����ᣩ=
| 26.10+26.08+26.12 |
| 3 |
HCl+NaOH=NaCl+H2O
0.0261L��0.1000mol/L 0.025L��C��NaOH��
��C��NaOH��=
| 0.0261L��0.1000mol/L |
| 0.025L |
�ʴ�Ϊ��0.1044mol/L��
��6��ѡ���̪��ָʾ�����ζ��յ�ʱ��Һ��ɫ��dz��ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ���ʴ�Ϊ����Һ��ɫ��dz��ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
������������Ҫ�����к͵ζ�����ȷ�ζ�������ָʾ����ѡ�����ݴ������������ǽ����Ĺؼ���ע������к͵�ʵ�ʣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
����˵������ȷ���ǣ�������
| A�������ڷ���һЩ�ر����ɫֲ����������ڿ����ľ��� |
| B���Ͻ���۵�һ����ڸ���ɳɷֽ������۵� |
| C������������Һ�ø��������Լ�ƿʢװ |
| D����ҵ���������ʴ���� |
�����������ͬ��ijֲ���Ӫ��Һ�����䷽���±���ʾ����������Ӫ��Һ�ijɷ֣�����˵���У���ȷ���ǣ�������
| KCl | K2SO4 | ZnSO4 | ZnCl2 | |
| 1 | 0.3mol | 0.2mol | 0.1mol | - |
| 2 | 0.1mol | 0.3mol | - | 0.1mol |
| A��ֻ��n��K+����ͬ |
| B��ֻ��n��Cl-����ͬ |
| C�������ӵ����ʵ�������ͬ |
| D�������ӵ����ʵ�����ȫ��ͬ |
���н���ʵ������ķ�Ӧ����ʽ����ȷ���ǣ�������
| A��SO2ͨ�뵽�μӷ�̪��NaOH��Һ�У���ɫ��dz��SO2+2NaOH=Na2SO3+H2O |
| B����Na2S������Hg2+��ˮ��Hg2++S2-�THgS�� |
| C����Ũ�ȵ�NH4Al��SO4��2��Һ��Ba��OH��2��Һ��1��2����Ȼ�ϳ��ְ�ɫ������NH4++Al3++2SO42-+2Ba2++4OH-=2BaSO4��+Al��OH��3��+NH3?H2O |
| D����Na2CO3��Һ����ˮ���е�CaSO4��Ca2++CO32-�TCaCO3�� |
����ͼ�������ȷ���ǣ�������
A�� Ũ�����ϡ�� |
B�� �����������������п�۷�Ӧ |
C�� ������������Ӧ�е������仯 |
D�� ��̬�⻯��е� |
 ��ʾ�ķ���ʽ
��ʾ�ķ���ʽ �еĺ��������ŵ�����Ϊ
�еĺ��������ŵ�����Ϊ

