��Ŀ����
������ʵ�����ӷ���ʽ�����Ӧ��ϵ����ȷ���ǣ���������
| A������۵⻯����Һ�еμ�ϡ���ᣬ�ڿ����з���һ��ʱ�����Һ������4H++4I-+O2�T2I2+2H2O | ||||
| B����K2Cr2O7��Һ�еμ�����Ũ���ᣬ��Һ��Ϊ��ɫ��Cr2O72-����ɫ��+H2O?2CrO42-����ɫ��+2H+ | ||||
| C��0.01mol?L-1 NH4Al��SO4��2��Һ��0.02mol?L-1 Ba��OH��2��Һ���������а�ɫ�������ɣ�Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+AlO2-+2H2O | ||||
D����ͭ�缫��ⱥ��ʳ��ˮ��2Cl-+2H2O
|
���㣺���ӷ���ʽ����д
ר�⣺���ӷ�Ӧר��
������A��������Һ�е����ӱ���������Ϊ�ⵥ�ʣ��������۱���ɫ��
B��Cr2O72-����ɫ��+H2O?2CrO42-����ɫ��+2H+������Ũ���ᣬƽ��������У���Һ�ʳ�ɫ��
C��0.01mol?L-1 NH4Al��SO4��2��Һ��0.02mol?L-1 Ba��OH��2��Һ�������ϣ�������ȫ��������������ȫ��������笠�ǡ������һˮ�ϰ���
D��ͭ�缫��⣬������ͭʧ���ӣ�
B��Cr2O72-����ɫ��+H2O?2CrO42-����ɫ��+2H+������Ũ���ᣬƽ��������У���Һ�ʳ�ɫ��
C��0.01mol?L-1 NH4Al��SO4��2��Һ��0.02mol?L-1 Ba��OH��2��Һ�������ϣ�������ȫ��������������ȫ��������笠�ǡ������һˮ�ϰ���
D��ͭ�缫��⣬������ͭʧ���ӣ�
���
�⣺A��������Һ�е����ӱ���������Ϊ�ⵥ�ʣ��������۱���ɫ��4H++4I-+O2�T2I2+2H2O����A��ȷ��
B��Cr2O72-����ɫ��+H2O?2CrO42-����ɫ��+2H+����K2Cr2O7��Һ�еμ�����Ũ���ᣬƽ��������У���Һ��Ϊ��ɫ����B����
C��0.01mol?L-1 NH4Al��SO4��2��Һ��0.02mol?L-1 Ba��OH��2��Һ�������ϣ�������ȫ��������������ȫ��������笠�ǡ������һˮ�ϰ���NH4++Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+Al��OH��3��+NH3?H2O����C����
D��ͭ�缫��⣬������ͭʧ���ӣ�Cu+2H2O
Cu��OH��2��+H2������D����
��ѡA��
B��Cr2O72-����ɫ��+H2O?2CrO42-����ɫ��+2H+����K2Cr2O7��Һ�еμ�����Ũ���ᣬƽ��������У���Һ��Ϊ��ɫ����B����
C��0.01mol?L-1 NH4Al��SO4��2��Һ��0.02mol?L-1 Ba��OH��2��Һ�������ϣ�������ȫ��������������ȫ��������笠�ǡ������һˮ�ϰ���NH4++Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+Al��OH��3��+NH3?H2O����C����
D��ͭ�缫��⣬������ͭʧ���ӣ�Cu+2H2O
| ||
��ѡA��
���������⿼�������ӷ���ʽ��д������ע�����⣬��Ҫ�ǻ�ѧƽ���ƶ�����������ԭ��Ӧ�жϣ�ע�����ӷ�Ӧ˳��͵��ԭ����Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
�����������ʼ䷢����Ӧ�����ĵ����ʵ������ᣬ�������������ǣ�������
| A��ľ̿��Ũ���� |
| B��ͭ��ϡ���� |
| C��п��ϡ���� |
| D��ľ̿��Ũ���� |
�����£����и���������ָ����Һ���ܴ���������ǣ�������
| A����pH=0����Һ�У�Cl-��Na+��K+��C6H5-O-��C6H5һΪ������ |
| B��������м�������ų�����Һ�У�NH4+��K+��HCO3-��Br- |
| C����H2SO3��Һ�У�HSO3-��K+��Cl-��Ba2+ |
| D���������ǣ�C6H12O6����Һ�У�SO42-��MnO4-��K+��H+ |
������ͼ�ס��ҡ����������ֻ�ѧʵ��������װ�ã����в����У�����ʵ��Ҫ����ǣ�������


| A����װ�ü��ռ�CH4����ȡb�˽���a�˳����ķ�ʽ |
| B����װ�����ռ�NO2���� |
| C����װ�ñ�����NH3 |
| D����������ȷ��ȡ25.00mL����KMnO4��Һ |
���з�Ӧ�����ӷ���ʽ��ȷ���ǣ�������
| A����AgNO3��Һ�еμӰ�ˮ��������Ag++NH3?H2O�TAgOH��+NH4+ |
| B����Mg��OH��2����Һ�еμ�FeCl3��Һ��3Mg��OH��2+2Fe3+�T2Fe��OH��3+3Mg2+ |
| C����Na2S2O3��Һ�м�������ϡ���2S2O32-+4H+�TSO42-+3S��+2H2O |
D��������Һ��ͨ������CO2���壺 +CO2+H2O��2 +CO2+H2O��2 +CO32- +CO32- |
����˵����ȷ���ǣ�������
| A�����к��ȵIJⶨʵ���У�������������ҺѸ�ٵ���ʢ����������ȼ��У�������������¼��Һ����ʼ�¶� |
B����ͼ��һ��ʱ�����ձ��ڵ���Һ�м����ɫ��K3[Fe��CN��6]��Һ���ɿ���Fe�缫��������ɫ�������� |
| C���ñ�����ζ�����NaOH��Һ��ˮϴ�����ʽ�ζ���δ����Һ��ϴ����ⶨ���ƫ�� |
| D���ü��ȵķ�ʽ����ȡNH4Cl�����л��е������ĵ� |
��NAΪ�����ӵ�������ֵ������˵����ȷ���ǣ�������
| A��1mol?L-1 Na2CO3��Һ�У���CO32-��ĿС��NA |
| B����״���£�11.2L O2��O3��ɵĻ�����庬��ԭ����ΪNA |
| C��14 g����ϩ��۱�ϩ�Ļ�����C-H������ĿΪ2NA |
| D�����³�ѹ�£�22.4L CO2������Na2O2��Ӧת�Ƶ�����ΪNA |
��ѧ����ᡢ���������������أ�����˵����ȷ���ǣ�������
| A��ʯӢֻ�������������ά |
| B���Ӻ�ˮ��ȡ���ʶ�����ͨ����ѧ��Ӧ����ʵ�� |
| C��Ϊ������ʳ���Ӫ���ɷ֣����Դ���ʹ��ʳƷ���Ӽ� |
| D�����ۡ���ά�غ�֬����һ�������¶����Է���ˮ�ⷴӦ |
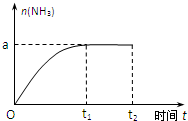 ��֪N2��g��+3H2��g��?2NH3��g������H=-92.4kJ?mol-1��
��֪N2��g��+3H2��g��?2NH3��g������H=-92.4kJ?mol-1��