��Ŀ����
���������
a��HO��CH2��3COOH b��HO��CH2��2OH c��CH3CH=CH-CNd��CH3CH2CH��CH3��COOH e��HOOC��CH2��4COOH
��1���ɷ����Ӿ۷�Ӧ�Ļ������� ���Ӿ���Ľṹ��ʽΪ ��
��2��ͬ�ַ��Ӽ�ɷ������۷�Ӧ���������Ļ������� ��������Ľṹ��ʽΪ ��
��3�����ַ��Ӽ�ɷ������۷�Ӧ���������Ļ������� �� ��������Ľṹ��ʽΪ ��
a��HO��CH2��3COOH b��HO��CH2��2OH c��CH3CH=CH-CNd��CH3CH2CH��CH3��COOH e��HOOC��CH2��4COOH
��1���ɷ����Ӿ۷�Ӧ�Ļ�������
��2��ͬ�ַ��Ӽ�ɷ������۷�Ӧ���������Ļ�������
��3�����ַ��Ӽ�ɷ������۷�Ӧ���������Ļ�������
���㣺�л���Ľṹ������
ר�⣺
������CH3CH=CH-CN����̼̼˫�����ɷ����Ӿ۷�Ӧ��HO��CH2��3COOH�����Ȼ����ǻ����������������۷�Ӧ��HO��CH2��2OH��HOOC��CH2��4COOH�ɷ������۷�Ӧ���Դ˽����⣮
���
�⣺��1��CH3CH=CH-CN����̼̼˫�����ɷ����Ӿ۷�Ӧ���Ӿ���Ϊ ���ʴ�Ϊ��c��
���ʴ�Ϊ��c�� ��
��
��2��HO��CH2��3COOH�����Ȼ����ǻ����������������۷�Ӧ�����۲���Ϊ ���ʴ�Ϊ��a��
���ʴ�Ϊ��a�� ��
��
��3��HO��CH2��2OH��HOOC��CH2��4COOH�ɷ������۷�Ӧ������Ϊ ��
��
�ʴ�Ϊ��b��e�� ��
��
 ���ʴ�Ϊ��c��
���ʴ�Ϊ��c�� ��
����2��HO��CH2��3COOH�����Ȼ����ǻ����������������۷�Ӧ�����۲���Ϊ
 ���ʴ�Ϊ��a��
���ʴ�Ϊ��a�� ��
����3��HO��CH2��2OH��HOOC��CH2��4COOH�ɷ������۷�Ӧ������Ϊ
 ��
���ʴ�Ϊ��b��e��
 ��
��
���������⿼���л���Ľṹ�����ʣ�Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬ע������л���Ĺ����ŵ����ʣ�����ע�ⷢ���Ӿ۷�Ӧ�����۷�Ӧ���ص㣬�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
���и���Ƚ��У�ǰ�ߴ��ں��ߵ��ǣ�������
| A���к������pH����ȵĴ�����Һ�����������ĵ�NaOH������ |
| B����ˮ��Ӳˮ��Ca2+��Mg2+�ĺ��� |
| C�����Ṥҵ�ж�������Ĵ�������Ӧ��ϳɰ������������õ�ѹǿ |
| D����ͬ�¶ȡ���ͬŨ�ȵ�NH4Cl��NH4HSO4��Һ��c��NH4+�� |
��ͼ��ʾ��ʵ���������Ƶı�ע��ʵ�������ȷ���ǣ�������
A��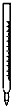 ��ʽ�ζ��� |
B�� ��������ȡ |
C��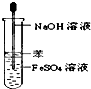 Fe��OH��2����ȡ |
D��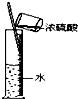 Ũ����ϡ�� |
ʵ����Ϊ�궨һ���ռ���Ʒ�����ʵ���Ũ�ȣ���ȡ���ռ���Ʒ4.0g���1L��Һ��ȡ��20.00mL������ƿ�У��Լ���Ϊָʾ������0.10mol?L-1 HCl����Һ�ζ���ǡ����ȫ�к�ʱ���ı�����19.00mL�����ռ���ֻ����һ�����ʣ������ǣ�������
| A��NaCl |
| B��Ca��OH��2 |
| C��Na2 CO3 |
| D��NaHCO3 |
��һ���¶��£�һ��������ܱ�������������ƽ�⣺H2������+I2������?2HI������
��֪H2��I2����ʼŨ�Ⱦ�Ϊ0.01mol?L-1ʱ����ƽ��ʱHI��Ũ��Ϊ0.16mol?L-1����H2��I2����ʼŨ�Ⱦ���Ϊ0.20mol?L-1����ƽ��ʱH2��Ũ�ȣ�mol?L-1���ǣ�������
��֪H2��I2����ʼŨ�Ⱦ�Ϊ0.01mol?L-1ʱ����ƽ��ʱHI��Ũ��Ϊ0.16mol?L-1����H2��I2����ʼŨ�Ⱦ���Ϊ0.20mol?L-1����ƽ��ʱH2��Ũ�ȣ�mol?L-1���ǣ�������
| A��0.16 | B��0.08 |
| C��0.04 | D��0.02 |
�����йؽ���ұ����˵���д�����ǣ�������
| A�������м��뽹̿�������� |
| B��������������������� |
| C������������м������ʯ�Խ������������۵� |
| D����ұ���ʪ��ұ���ܺĴ� |
