��Ŀ����
17��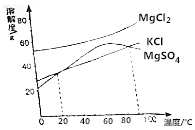 �ҹ�ʳ����80%���Ե��¾��κ����Σ����ξ�ˮɹ������ȡʳ�εij��÷�����
�ҹ�ʳ����80%���Ե��¾��κ����Σ����ξ�ˮɹ������ȡʳ�εij��÷�������1����ȥ��ˮ�������IJ����ǹ��ˣ�����ˮɹ�ε�ԭ���������ᾧ��
��2��ɹ��ʣ���±ˮ��Ҫ����KCl��MgCl2��MgSO4�ȣ���ͼ�Ǽ������ʵ��ܽ�����ߣ���ش��������⣮
������±ˮ���ȵ�90��������������ˮ�֣�����������Ҫ��MgSO4��ԭ���Ǽ��ȵ�90�����ϣ�MgSO4���ܽ�������¶����߶���С��
������±ˮ��к�����ȴ������KCl������¶ȷ�Χ��20��90�棻
��3��Ϊ�����Ƶ�ʳ�����Ƿ���Mg2+���ɽ��������������Һ�������еμ�NaOH���йط�Ӧ�����ӷ���ʽ��2OH-+Mg2+=Mg��OH��2����
���� ��1�������Һ������ù��ˣ�����Һ����ȡ�����������ᾧ�IJ�����
��2���ٸ���ͼ��90����MgSO4���ܽ�ȱ仯������
������KCl����ʱ��MgCl2��MgSO4���ܽ��Ҫ����KCl��
��3������þ�������������ƣ������ɰ�ɫ������
��� �⣺��1����ȥ��ˮ���������Ƿ����Һ���������Ϊ���ˣ���ˮɹ�Σ�����Һ����ȡ�����Ե����ʣ������Ϊ�����ᾧ��
�ʴ�Ϊ�����ˣ������ᾧ��
��2������ͼ��90����MgSO4���ܽ�ȱ仯��֪�����ȵ�90�����ϣ�MgSO4���ܽ�������¶����߶���С����KCl��MgCl2���ܽ���������Խ�±ˮ���ȵ�90��������������ˮ�֣������γɱ�����Һ����MgSO4����MgSO4����������
�ʴ�Ϊ��MgSO4�����ȵ�90�����ϣ�MgSO4���ܽ�������¶����߶���С��
������KCl����ʱ��MgCl2��MgSO4���ܽ��Ҫ����KCl����ͼ���֪����20��90��ʱ��KCl���ܽ��С�������������ʵ��ܽ�ȣ���������KCl������¶ȷ�Χ��20��90�棻
�ʴ�Ϊ��20��90��
��3��Ϊ�����Ƶ�ʳ�����Ƿ���Mg2+���ɽ��������������Һ��Ȼ���������������Һ���а�ɫ��������˵������þ���ӣ��䷴Ӧ�����ӷ���ʽΪ2OH-+Mg2+=Mg��OH��2����
�ʴ�Ϊ��NaOH��2OH-+Mg2+=Mg��OH��2����
���� ���⿼�������ʷ����ᴿ��ʵ�鷽����ơ����ӷ���ʽ����д���ܽ�����¶ȵĹ�ϵͼ����Ŀ�ۺ��Խ�ǿ���Ѷ��еȣ������ڿ���ѧ���ķ���������ʵ��̽��������ע������������������ʵ��ܽ�����¶ȵĹ�ϵ��

 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�| A�� | ����Һ�ĵ���������pHֵ����ͬ | |
| B�� | �к�����Һ������NaOH�����ʵ�����ͬ | |
| C�� | �ֱ���������Zn��ȫ��Ӧ��������������ʿ죬������ | |
| D�� | �����������10mL��Ϻ�10mL 0.1mol•L-1��NaOH������c��H+��=c��CH3COO-�� |
| A�� | CH4��C2H4 | B�� | C2H2��C2H4 | C�� | C2H4��C2H6 | D�� | C4H8��C3H6 |
| A�� | 25��ʱ��1LpH=2��HCl��Һ�У���ˮ�������H+����Ŀ0.01NA | |
| B�� | ��״���£�2.24L��CCl4�к��е���ԭ����Ϊ0.4NA | |
| C�� | �����£�1molCO2�к��еĹ��õ��Ӷ���ĿΪ2NA | |
| D�� | ��״���£�2.24L Cl2��ˮ��ַ�Ӧ��ת�Ƶĵ�����С��0.1NA |

�������ʵ�����������£�
| �۵�/�� | �е�/�� | ��ע | |
| ���� | 44 | 280.5 | |
| PH3 | -133.8 | -87.8 | ������ˮ���л�ԭ�� |
| SiF4 | -90 | -86 | ��ˮ�� |
��1����������ʯ����Ҫ����;�����������ϣ�Լռ��ʯʹ������69%��
��2������ʯΪԭ�ϣ�ʪ�����������Ca5F��PO4��3��Ӧ�Ļ�ѧ����ʽΪ��Ca5F��PO4��3+5H2SO4=3H3PO4+5CaSO4+HF��������1t�ۺϺ���P2O5Լ30%����ʯ�������Ƶõ�85%����Ʒ����0.49t��
��3����ͼ��b����ʾ���ȷ������������̵ĵ�һ���ǽ�SiO2��������̿����ʯ��ϣ����·�Ӧ���ɰ��ף�¯������Ҫ�ɷ���CaSiO3���ѧʽ����������1����Ҫ��������Һ̬���ף�������2����Ҫ�������ǹ�̬���ף�
��4��β������Ҫ����SiF4��CO��������������PH3��H2S��HF�ȣ���β����ͨ�봿����Һ���ɳ�ȥSiF4��H2S��HF����ͨ�����������Һ���ɳ�ȥPH3�����ѧʽ����
��5�������ʪ�����ᣬ�ȷ����Ṥ�ո��ӣ��ܺĸߣ����ŵ��Dz�Ʒ���ȸߣ�
 ��ͼ�����Թ�a���ȼ���3mL���Ҵ�����ҡ��������2mLŨ���ᣬ�ټ���2mL��ˮ���ᣬ�ò�������ֽ�����Թ̶ܹ�������̨�ϣ����Թ�b�м�����������̼������Һ�����Ӻ�װ�ã��þƾ��ƶ��Թܼ��ȣ����۲쵽�Թ�b������������ʱֹͣʵ�飮
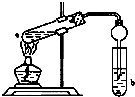
��ͼ�����Թ�a���ȼ���3mL���Ҵ�����ҡ��������2mLŨ���ᣬ�ټ���2mL��ˮ���ᣬ�ò�������ֽ�����Թ̶ܹ�������̨�ϣ����Թ�b�м�����������̼������Һ�����Ӻ�װ�ã��þƾ��ƶ��Թܼ��ȣ����۲쵽�Թ�b������������ʱֹͣʵ�飮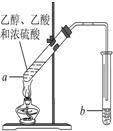 �����Ѿ��ܷⴢ��ʱ��Խ��������Խ�ã�ԭ��֮һ�Ǵ������������������ζ��������ʵ����Ҳ��������ͼ��ʾ��װ����ȡ������������ش��������⣮
�����Ѿ��ܷⴢ��ʱ��Խ��������Խ�ã�ԭ��֮һ�Ǵ������������������ζ��������ʵ����Ҳ��������ͼ��ʾ��װ����ȡ������������ش��������⣮