��Ŀ����
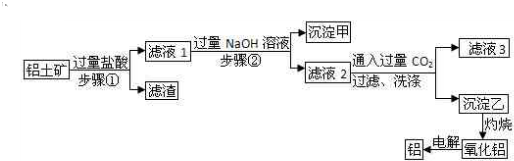
���Ͻ���һ����Ҫ�������ܲ��ϣ��¹��С����á�����еġ������������벻��������ҵ������������Ҫ�ɷ���Al2O3��������SO2��MgO��Fe2O3�����ʣ�ұ��������Ҫ��������ͼ��ʾ��
����������Ϣ�ش��������⣺
��1��д����������漰Al2O3�����ӷ���ʽ ����������Ҫ�ɷ֣� ���ѧʽ����
��2�����������õ����ι��˲��������˹������õ��IJ�����������©�����ձ�֮�⣬���� ��ϴ�ӳ����IJ�������Ϊ ��
��3��д����Һ2ͨ�������CO2���Ļ�ѧ����ʽ�� ��������Һ3�������ӵķ���Ϊ ��
��4����ҵ�ϲ��õ��װ����ȡ���ý�����������������������ȫ����ʯī�缫��Ӧ����CO��CO2���壬��������ÿ����8.1g��Al��������ʧʯī3.0g���������ϲ���CO�����ʵ����� ��

����������Ϣ�ش��������⣺
��1��д����������漰Al2O3�����ӷ���ʽ
��2�����������õ����ι��˲��������˹������õ��IJ�����������©�����ձ�֮�⣬����
��3��д����Һ2ͨ�������CO2���Ļ�ѧ����ʽ��
��4����ҵ�ϲ��õ��װ����ȡ���ý�����������������������ȫ����ʯī�缫��Ӧ����CO��CO2���壬��������ÿ����8.1g��Al��������ʧʯī3.0g���������ϲ���CO�����ʵ�����
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��,����ұ����һ��ԭ��,�����Ļ����뻷������Դ����
ר�⣺ʵ�������
��������ҵ������������Ҫ�ɷ���Al2O3��������SO2��MgO��Fe2O3�����ʣ��������̿�֪���ӹ��������ܽ��MgO��Fe2O3��Al2O3��HCl��Ӧ�ܽ⣬��SiO2��HCl����Ӧ�������ܽ⣬����ΪSiO2��Ȼ����Һ1�м����ռ���ɳ�����ΪFe��OH��3��Mg��OH��2����Һ2�д������ڵ���������AlO2-��Cl-��OH-��ͨ�������̼��Ӧ���ɳ�����ΪAl��OH��3����Һ3�к�NaCl��NaHCO3�����������ֽ��������������������������Al���Դ������
���
�⣺��ҵ������������Ҫ�ɷ���Al2O3��������SO2��MgO��Fe2O3�����ʣ��������̿�֪���ӹ��������ܽ��MgO��Fe2O3��Al2O3��HCl��Ӧ�ܽ⣬��SiO2��HCl����Ӧ�������ܽ⣬����ΪSiO2��Ȼ����Һ1�м����ռ���ɳ�����ΪFe��OH��3��Mg��OH��2����Һ2�д������ڵ���������AlO2-��Cl-��OH-��ͨ�������̼��Ӧ���ɳ�����ΪAl��OH��3����Һ3�к�NaCl��NaHCO3�����������ֽ��������������������������Al��
��1����������漰Al2O3�����ӷ���ʽΪAl2O3+6H+=2Al3++3H2O�����ɳ�����ΪFe��OH��3��Mg��OH��2��
�ʴ�Ϊ��Al2O3+6H+=2Al3++3H2O��Fe��OH��3��Mg��OH��2��
��2��������Ҫ�ձ���©�����������ȣ�ϴ�ӳ����IJ�������Ϊ���������ע��ˮ��û������ʹˮ��Ȼ���£��ظ�2��3�Σ�
�ʴ�Ϊ�������������������ע��ˮ��û������ʹˮ��Ȼ���£��ظ�2��3�Σ�
��3����Һ2ͨ�������CO2�Ļ�ѧ����ʽΪNaAlO2+CO2+2H2O=Al��OH��3��+NaHCO3��������Һ3�������������ӵķ���Ϊ��ɫ��Ӧ��
�ʴ�Ϊ��NaAlO2+CO2+2H2O=Al��OH��3��+NaHCO3����ɫ��Ӧ��
��4��n��Al��=
=0.3mol��n��C��=
=0.25mol����CO��CO2�����ʵ���Ϊx��y��
�ɵ����غ��ԭ���غ��֪��
�����x=0.05mol��
�����ϲ���CO�����ʵ�����0.05mol��
�ʴ�Ϊ��0.05mol��
��1����������漰Al2O3�����ӷ���ʽΪAl2O3+6H+=2Al3++3H2O�����ɳ�����ΪFe��OH��3��Mg��OH��2��
�ʴ�Ϊ��Al2O3+6H+=2Al3++3H2O��Fe��OH��3��Mg��OH��2��
��2��������Ҫ�ձ���©�����������ȣ�ϴ�ӳ����IJ�������Ϊ���������ע��ˮ��û������ʹˮ��Ȼ���£��ظ�2��3�Σ�
�ʴ�Ϊ�������������������ע��ˮ��û������ʹˮ��Ȼ���£��ظ�2��3�Σ�
��3����Һ2ͨ�������CO2�Ļ�ѧ����ʽΪNaAlO2+CO2+2H2O=Al��OH��3��+NaHCO3��������Һ3�������������ӵķ���Ϊ��ɫ��Ӧ��
�ʴ�Ϊ��NaAlO2+CO2+2H2O=Al��OH��3��+NaHCO3����ɫ��Ӧ��
��4��n��Al��=
| 8.1g |
| 27g/mol |
| 3g |
| 12g |
�ɵ����غ��ԭ���غ��֪��
|
�����ϲ���CO�����ʵ�����0.05mol��
�ʴ�Ϊ��0.05mol��
���������⿼����������ᴿ���ۺ�Ӧ�ã�Ϊ��Ƶ���㣬�������̼������з�Ӧ���������뷽����Ϊ���Ĺؼ������ط�����ʵ���������ۺϿ��飬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 �������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������
�������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������| a | b | c | |
| A | Al | AlCl3 | Al��OH��3 |
| B | HNO3 | NO | NO2 |
| C | Si | SiO2 | H2SiO3 |
| D | Na2O | Na2CO3 | NaHCO3 |
| A��A | B��B | C��C | D��D |
���б���ʽ������ǣ�������
| A��H��Cl | ||
B�� | ||
C��
| ||
| D��O=C=O |
 ij�¶��£���ͬpH������ʹ�����Һ�ֱ��ˮϡ�ͣ�pH����Һ����仯��������ͼ��ʾ�������ж���ȷ���ǣ�������
ij�¶��£���ͬpH������ʹ�����Һ�ֱ��ˮϡ�ͣ�pH����Һ����仯��������ͼ��ʾ�������ж���ȷ���ǣ�������| A����ͬ���ʱ��c����Һ�кͼ����������a�� |
| B��b����Һ�ĵ����Ա�c����Һ�ĵ�����ǿ |
| C��b�����Ũ�ȴ���a�����Ũ�� |
| D����Ϊ����ϡ��ʱ��pH�仯���� |
���ֶ�����Ԫ��W��X��Y��Zԭ����������������ԭ�ӵ�����������֮��Ϊ19��W��XԪ��ԭ����������֮��Ϊ1��2��X2+��Z-���ӵĵ�����֮��Ϊ8������˵����ȷ���ǣ�������
| A����W���ڵ�ͬ����Ԫ�ص��ʵ���Ҫ��;���������� |
| B��X���ʲ������û���W���� |
| C��Ԫ��ԭ�Ӱ뾶�Ӵ�С��˳����X��Y��Z |
| D���ɷǽ�����ǿ����֪����������W�ĺ������Ʊ�Z�ĺ����� |
X��Y��Z��M��W���ֶ�����Ԫ�أ�X��������������Ӳ�����ͬ��Y��Z��M��W�����ڱ��е����λ�����±�����Wԭ�Ӻ����������Mԭ��������������2��������˵������ȷ���ǣ�������

| A��ԭ�Ӱ뾶��W��Y��Z��M��X |
| B��X��Y��Z ����Ԫ���γɵĻ������п��ܼ������Ӽ����й��ۼ� |
| C��W�ֱ���M��ZԪ���γɵĻ�����WM4��WZ2����ԭ�Ӿ��� |
| D��X�ֱ���Y��Z��M��W�γɵij����������У��ȶ�����õ���XM���е�X2Z��XM |
����ͼʾ���Ӧ������������ǣ�������
A��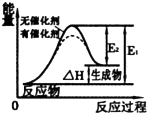 ͼ��ʾ�����ܸı仯ѧ��Ӧ���ʱ� |
B��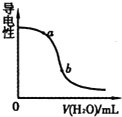 ͼ��ʾ��ˮ�м�ˮʱ��Һ�����Եı仯���������Һc��OH-����С��a��b |
C��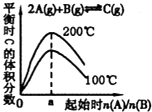 ��ͼ��֪��Ӧ2A��g��+B��g��?C��g���ġ�H��O���� a=2 |
D��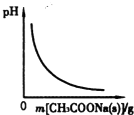 ͼ��ʾ��CH3COOH��Һ������CH3COONa�������ҺpH�ı仯��� |


