��Ŀ����
14��ˮ��ʯ��IDHs����һ������ǽ������ϣ����������ϵ���ȼ���ȣ�ij����С���ͬѧͨ����ͼ�����Ʊ�þ��ˮ��ʯ��
��ˮ��ʯ���Ʊ�
��1��A��ҺΪһ��Ũ�ȵ�Mg��NO3��2��Al��NO3��2�Ļ����Һ������ʱ�����ձ����������⣬����Ҫ�IJ���������500 mL����ƿ����ͷ�ιܣ�
��2��B��ҺΪc��NaOH��=1.6mol•L-1��c��Na2CO3��=0.8mol•L-1�Ļ����Һ��������ʱ�����ȡNaOH������Ϊ32.0g��B��Һ��c��Na+��=3.2mol•L-1��
��3������ʱ��������������������
��ˮ��ʯ��ɲⶨ
��4���������Ƶõ�ˮ��ʯ���л�ѧ��������ˮ��ʯ���ΪMgaAlb��OH��c��CO3��d����֪a+b+c+d=25��a��b��c��dΪ�������������Ƶõ���Ʒ[��0.1molMgaAlb��OH��c��CO3��d]��1mol•L-1����ʹ����ȫ�ܽ⣮��
������������������Ϊ��0.2a+0.3b��L���ú���ĸ����ʽ��ʾ����
�����μӷ�Ӧ��HCl�����ʵ�������CO2�����ʵ���֮��Ϊ18��1�����ˮ��ʯ�Ļ�ѧʽΪMg6Al2��OH��16CO3��
���� ��1����������һ�����ʵ���Ũ�ȵ���Һ�IJ���ѡ��ʹ�õ�������
��2������n=cV����NaOH��������c=$\frac{n}{V}$����B��Һ��c��Na+����
��3������ʱ��������������������
��4���پݷ���ʽ��MgaAlb��OH��c��CO3��d+��2a+3b��HCl=bAlCl3+aMgCl2+��a+$\frac{3}{2}$b+$\frac{1}{2}$c��H2O+dCO2���������������
�ڸ��ݲμӷ�Ӧ����������ʵ���������CO2�����ʵ���֮��Ϊ18��1�����ϼ۴�����Ϊ0����֪��ϵ�з�������м��㣮
��� �⣺��1������500mL һ��Ũ�ȵ�Mg��NO3��2��Al��NO3��2�Ļ����Һ�IJ���Ϊ�����㡢�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ���Ҫʹ�õ������У�Կ�ס���ƽ���ձ���500mL����ƿ����ͷ�ιܡ��������ȣ����Ի�ȱ�ٵIJ�������Ϊ��500 mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��500 mL����ƿ����ͷ�ιܣ�
��2����ΪB��ҺΪc��NaOH��=1.6mol•L-1��c��Na2CO3��=0.8mol•L-1�Ļ����Һ����NaOH������m=40n=40cV=40��1.6��0.5=32.0g��c��Na+��=$\frac{n}{V}$=$\frac{1.6��0.5+0.8��0.5��2}{0.5}$=3.2mol•L-1���ʴ�Ϊ��32.0g��3.2��
��3������ʱ���������������������ʴ�Ϊ��������
��4���پݷ���ʽ��MgaAlb��OH��c��CO3��d+��2a+3b��HCl=bAlCl3+aMgCl2+��a+$\frac{3}{2}$b+$\frac{1}{2}$c��H2O+dCO2����
1 ��2a+3b�� d
1molˮ��ʯ���ģ�2a+3b��mol���ᣬ��0.1molˮ��ʯ���ģ�0.2a+0.3b��mol���ᣬ�����������Ϊ��$\frac{��0.2a+0.3b��mol}{1mol/L}$=��0.2a+0.3b��L���ʴ�Ϊ����0.2a+0.3b����
���ֲμӷ�Ӧ����������ʵ���������CO2�����ʵ���֮��Ϊ18��1������$\frac{2a+3b}{d}$=18��1
�ݻ��ϼ۴�����Ϊ0�����У�2a+3b=c+2d
����a+b+c+d=25
�ʽ�ã�a��b��c��d=6��2��16��1����ˮ��ʯ�Ļ�ѧʽΪ��Mg6Al2��OH��16CO3��
�ʴ�Ϊ��Mg6Al2��OH��16CO3��
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ���������㣬����������ѧ���ķ��������⼰��ѧ������������ȷ����ԭ�������ǽ���ؼ���ע�������ʽ��Ӧ�ã���Ŀ�ѶȲ���

| A�� | �÷�Ӧ�ġ�S��0 | B�� | �÷�Ӧ�ġ�H��0 | ||
| C�� | �÷�Ӧ�Dz����淴Ӧ | D�� | �÷�Ӧ���ﲻ����ɴ�����Ⱦ |
| A�� | c��Na+����c��HCO3-����c��H+����c��OH-�� | B�� | c��Na+��+c��H+��=c��HCO3-��+c��OH-��+2c��CO32-�� | ||
| C�� | c��Na+��=c��HCO3-����c��OH-����c��H+�� | D�� | c��Na+��=c��HCO3-��+c��H2CO3��+c��CO32-�� |
| A�� | ������Һ | B�� | ������ | C�� | NaHCO3��Һ | D�� | ��̪��Һ |
 ��ijþ���Ͻ�Ͷ��һ�������ϡ�����У����Ͻ���ȫ�ܽ������Һ�����1mol/L��NaOH��Һ�����ɳ������ʵ��������NaOH��Һ����Ĺ�ϵ��ͼ��ʾ������˵���д�����ǣ�������
��ijþ���Ͻ�Ͷ��һ�������ϡ�����У����Ͻ���ȫ�ܽ������Һ�����1mol/L��NaOH��Һ�����ɳ������ʵ��������NaOH��Һ����Ĺ�ϵ��ͼ��ʾ������˵���д�����ǣ�������| A�� | C���Ӧ�ij���ΪMg��OH��2 | |
| B�� | Al������Ϊ27��b-a����10-3 | |
| C�� | �úϽ������ᷴӦ������H2Ϊ��a-x����10-3mol | |
| D�� | ��ͼ����ȷ��x��ȡֵ��ΧΪ��0��x����4a-3b�� |
��+1 ��+2 ��+3 ��+4��
| A�� | �ٻ�� | B�� | �ٻ�� | C�� | �ڻ�� | D�� | �ڻ�� |
 ��ͼ�����ձ��еμӼ���Ũ���ᣬCaCO3���廹��ʣ�ࣨ����������¶ȵı仯����������ֵ��С���ǣ�������
��ͼ�����ձ��еμӼ���Ũ���ᣬCaCO3���廹��ʣ�ࣨ����������¶ȵı仯����������ֵ��С���ǣ�������| A�� | c��Ca2+�� | B�� | c��CO32-�� | C�� | c��H+�� | D�� | c��̼��Ƶ��ܽ�ȣ� |
 ç�����Ǻϳ����������е�ҩ��--��ƣ�Tamiflu����ԭ��֮һ��ç������A��һ���칹�壮A�Ľṹ��ʽ���£�
ç�����Ǻϳ����������е�ҩ��--��ƣ�Tamiflu����ԭ��֮һ��ç������A��һ���칹�壮A�Ľṹ��ʽ���£� �����䷴Ӧ��������ȥ��Ӧ����ѧ����ʽ��
�����䷴Ӧ��������ȥ��Ӧ����ѧ����ʽ�� $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$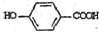 +2H2O��
+2H2O�� ��
��