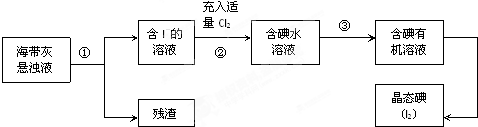
��Ŀ����
 ijͬѧ������100mL 0.10mol/L CuSO4��Һ�����²���1��5�������ƹ��̼�ʾ��ͼ��
ijͬѧ������100mL 0.10mol/L CuSO4��Һ�����²���1��5�������ƹ��̼�ʾ��ͼ������1��ȷ��ȡһ�������ĵ����� CuSO4?5H2O������������ˮ�ܽ⣮
����2����������Һת�Ƶ�����X�У�������ˮ��ϴ�ձ�
�Ͳ�����2��3�Σ���ϴ��ҺҲת�Ƶ�X�У�
����3��������X�м�ˮ��Һ����X�Ŀ̶���1��2cm����
��1������X��������
��2�����ڸ����ƹ��̣�����˵����ȷ����
A������1�У�Ӧ��ȡ����������Ϊ2.5g
B������2�У�ϴ��Һ����Ҫת�Ƶ�����X��
C������4��Ϊ���ݣ����ڸ�ͬѧ�۲췽������ȷ��������������ҺŨ��ƫ��
D������5ҡ�Ⱥ��ã�����Һ����ڿ̶��ߣ�Ӧ������ˮ����Һ����̶������У�
���㣺����һ�����ʵ���Ũ�ȵ���Һ
ר�⣺
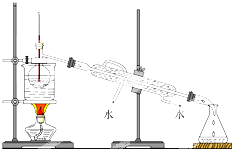
��������1�����������Ľṹ������ȷ�����ƣ�
��2��A������ͭ�����ʵ�����������ͭ��������ʵ���������m=cVM������Ҫ����ͭ�����������
B��ϴ��ʱ��ϴ��Һ��Ҫת�Ƶ�����ƿ�У�
C�����ӵ�����Һ���ƫ��Ũ��ƫС��
D������Һ����ڿ̶��ߣ�������ˮ����Һ����̶������У�����������ڿ̶������ϵ�Һ������䣬��Һ�����ƫ��
��2��A������ͭ�����ʵ�����������ͭ��������ʵ���������m=cVM������Ҫ����ͭ�����������
B��ϴ��ʱ��ϴ��Һ��Ҫת�Ƶ�����ƿ�У�
C�����ӵ�����Һ���ƫ��Ũ��ƫС��
D������Һ����ڿ̶��ߣ�������ˮ����Һ����̶������У�����������ڿ̶������ϵ�Һ������䣬��Һ�����ƫ��
���
�⣺��1����ʵ��������ƿ����100mL0.10mol?L-1��CuSO4��Һ������X��������100mL����ƿ��
�ʴ�Ϊ��100mL����ƿ��
��2��A������ͭ�����ʵ�����������ͭ��������ʵ���������100mL0.10mol?L-1��CuSO4��Һ����Ҫ����ͭ���������Ϊ��0.1L��0.10mol/L��250g/mol=2.5g����A��ȷ��
B��ϴ��ʱ��ϴ��Һ��Ҫת�Ƶ�����ƿ�У�ȷ�����ʵ��������䣬��B����
C��ͼʾΪ���Ӷ��ݣ����Ӷ��ݻᵼ����Һ���ƫ������������ҺŨ��ƫ�ͣ���C��ȷ��
D������Һ����ڿ̶��ߣ�������ˮ����Һ����̶������У�����������ڿ̶������ϵ�Һ������䣬��Һ�����ƫ������������ҺŨ��ƫ�ͣ���D����
�ʴ�Ϊ��AC��
�ʴ�Ϊ��100mL����ƿ��
��2��A������ͭ�����ʵ�����������ͭ��������ʵ���������100mL0.10mol?L-1��CuSO4��Һ����Ҫ����ͭ���������Ϊ��0.1L��0.10mol/L��250g/mol=2.5g����A��ȷ��
B��ϴ��ʱ��ϴ��Һ��Ҫת�Ƶ�����ƿ�У�ȷ�����ʵ��������䣬��B����
C��ͼʾΪ���Ӷ��ݣ����Ӷ��ݻᵼ����Һ���ƫ������������ҺŨ��ƫ�ͣ���C��ȷ��
D������Һ����ڿ̶��ߣ�������ˮ����Һ����̶������У�����������ڿ̶������ϵ�Һ������䣬��Һ�����ƫ������������ҺŨ��ƫ�ͣ���D����
�ʴ�Ϊ��AC��
���������⿼��һ�����ʵ���Ũ����Һ���ƣ���Ŀ�ѶȲ���ּ�ڿ���ѧ���Ի���֪ʶ���������գ�������Ϊ�ѵ㣬���Ը���ʵ�������c=
��Ӱ����з�����
| n |
| V |

��ϰ��ϵ�д�
�����Ŀ
�������ֱ����뷴Ӧ����ʽ��Ӧ����ȷ���ǣ�������
| A���������е���AgNO3��Һ�������е���Ԫ�أ�Br-+Ag+�TAgBr�� | ||||
| B���ô����ȥˮ����CaCO3+2H+�TCa2++H2O+CO2�� | ||||
C��������������Լ��ķ�Ӧ��CH2OH��CHOH��4CHO+2Cu��OH��2+OH-
| ||||
D����������Һ��ͨ������CO2��CO2+H2O+ �� �� +HCO3- +HCO3- |
Ԫ��X��Y��Zԭ������֮��Ϊ36��X��Y��ͬһ���ڣ�X+��Z2-������ͬ�ĺ�����Ӳ�ṹ�������Ʋⲻ��ȷ���ǣ�������
| A��ͬ����Ԫ����X�Ľ�������ǿ |
| B��ͬ����Ԫ����Y����ۺ������������ǿ |
| C��ԭ�Ӱ뾶X��Y�����Ӱ뾶X+��Z2- |
| D��ͬ��Ԫ����Z���⻯���ȶ������ |
һ���¶��£���2mol A2��x mol B2ͨ�����Ϊ2L���ܱ������з�����ӦA2��g��+3B2��g��?2AB3��g��������Ӧ�ﵽƽ��ʱ�����AB3�����ʵ���Ϊ2mol�����˷�Ӧ��ƽ�ⳣ��K=0.25����x��ֵ�ǣ�������
| A��7 | B��8 | C��9 | D��10 |
�����淴Ӧ��ƽ��ı䷴Ӧ��������仯������ȷ���ǣ�������
A�� I2��g��+H2��g��?2HI��g�� |
B�� CH3COOH?H++CH3COO-��������Һ����仯�� |
C��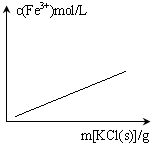 FeCl3+3KSCN?Fe��SCN��3+3KCl������Һ������仯�� |
D��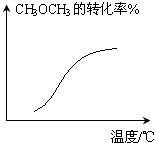 CH3OCH3��g��+3H2O��g��?6H2��g��+2CO��g��-Q����ѹ�� |
����˵����ȷ���ǣ�������
| A��7.1g����������������������Һ��Ӧת�Ƶĵ�����Ϊ0.2��6.02��1023 |
| B��V L a mol?L-1���Ȼ�����Һ�У���Fe3+����ĿΪ6.02��1023����Cl-����Ŀ����3��6.02��1023 |
| C����ҵ�õ�ⷨ���д�ͭ����ʱ��ÿת��1mol���ӣ��������ܽ��Cu����Ϊ0.5��6.02��1023 |
| D����״���£�22.4LNO��11.2L O2��Ϻ�����ķ�������Ϊ1.0��6.02��1023 |