��Ŀ����
19�����ֹ����к������гɷ֣�
��֪����

C2H4O2$��_{����}^{Br_{2}}$BrCH2COOH$��_{��}^{NaOHˮ��Һ}$D$\stackrel{�ữ}{��}$��
��1mol������NaHCO3�����ʵ����Ǽ�2��
��

�ش��������⣺
��1��������֪�ٵõ���
�ټ��к��в����ͼ��Ĺ���������Ϊ�Ȼ���
��A��BΪȡ����Ӧ��A�Ľṹ��ʽΪCH3COOH��
��B��D�Ļ�ѧ����ʽΪBrCH2COOH+2NaOH$��_{��}^{ˮ}$HOCH2COONa+NaBr+H2O��
��2������һ��������������״�����л��߷��ӻ�����Ļ�ѧ����ʽΪn HOOCCH��OH��COOH$\stackrel{һ������}{��}$
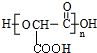 +��n-1��H2O��
+��n-1��H2O����3���ɱ�������;���ɵ�һ����Ҫ��ҽҩ�������м���J�����ַ�Ӧ������ȥ����

���û�ѧ������ȥE�в�����������������ʱE�ͱ���Һ̬������������ˮ�е��ܽ⣩����1�������Լ�������Ϊ����������ͭ��������Һ����2��3�����ֱ��ǹ��ˡ���Һ��
�ھ�E��G��H�����Ĺ��������ǻ������Ա����л��������д��ں��ֹ����ŵ������Ǻ�������ǣ�
��J��ͬ���칹�����ں˴Ź�����������ʾΪ����壬�������Ϊ3��2����״�Ҳ�����֧�����칹�干��8�֣����������칹��������ij�칹��L�еĹ����Ŷ�����H2�����ӳɷ�Ӧ����L�Ľṹ��ʽΪCH3CH2COC��CCOCH2CH3��CH3COCH2C��CCH2COCH3��ֻдһ�֣���
���� ��1����A�ķ���ʽ��B�Ľṹ��֪��A���巢��ȡ����Ӧ����B����AΪCH3COOH��B�ڼ��������·���ˮ�ⷴӦ���кͷ�Ӧ�õ�DΪHOCH2COONa��D�ữ�õ��ף�
��2��1mol������NaHCO3�����ʵ����Ǽ�2�������ҷ����к���2���Ȼ�������һ��������������״֬���л��߷��ӻ��������ΪHOOCCH��OH��COOH���õ��ĸ߷��ӻ�����Ϊ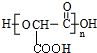 ��
��
��3��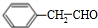 �����������ӳɷ�Ӧ����
�����������ӳɷ�Ӧ����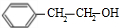 ��E����������Ӧ��������Ӧ�õ�G��G������Ϣ����ת���õ�HΪ
��E����������Ӧ��������Ӧ�õ�G��G������Ϣ����ת���õ�HΪ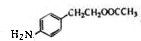 ��JΪ
��JΪ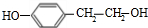 ��
��
��� �⣺��1����A�ķ���ʽ��B�Ľṹ��֪��A���巢��ȡ����Ӧ����B����AΪCH3COOH��B�ڼ��������·���ˮ�ⷴӦ���кͷ�Ӧ�õ�DΪHOCH2COONa��D�ữ�õ��ף�
�ټ��к��в����ͼ��Ĺ���������Ϊ���Ȼ����ʴ�Ϊ���Ȼ���
��A��BΪȡ����Ӧ��A�Ľṹ��ʽΪ��CH3COOH���ʴ�Ϊ��CH3COOH��
��B��D�Ļ�ѧ����ʽΪ��BrCH2COOH+2NaOH$��_{��}^{ˮ}$HOCH2COONa+NaBr+H2O��
�ʴ�Ϊ��BrCH2COOH+2NaOH$��_{��}^{ˮ}$HOCH2COONa+NaBr+H2O��
��2��1mol������NaHCO3�����ʵ����Ǽ�2�������ҷ����к���2���Ȼ�������һ��������������״֬���л��߷��ӻ��������ΪHOOCCH��OH��COOH���õ��ĸ߷��ӻ�����Ϊ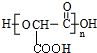 ����Ӧ����ʽΪ��n HOOCCH��OH��COOH$\stackrel{һ������}{��}$
����Ӧ����ʽΪ��n HOOCCH��OH��COOH$\stackrel{һ������}{��}$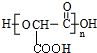 +��n-1��H2O��
+��n-1��H2O��
�ʴ�Ϊ��n HOOCCH��OH��COOH$\stackrel{һ������}{��}$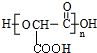 +��n-1��H2O��
+��n-1��H2O��
��3��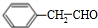 �����������ӳɷ�Ӧ����
�����������ӳɷ�Ӧ����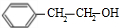 ��E����������Ӧ��������Ӧ�õ�G��G������Ϣ����ת���õ�HΪ
��E����������Ӧ��������Ӧ�õ�G��G������Ϣ����ת���õ�HΪ ��JΪ
��JΪ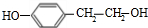 ��
��
���û�ѧ������ȥE�в���������������1�������Լ�������Ϊ����������ͭ��������Һ����2��3�������ֱ��ǹ��ˡ���Һ���ʴ�Ϊ������������ͭ��������Һ��
�ھ�E��G��H�����Ĺ��������ǻ������Ա����л��������д��ں��ֹ����ŵ������Ǻ�������ǣ�
�ʴ�Ϊ���ǻ�����������ǣ�
��J��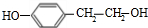 ����ͬ���칹�����ں˴Ź�����������ʾΪ����壬�������Ϊ3��2����״�Ҳ�����֧�����칹�壬�����к���2��-CH3��2��-CH2-����Ϊ�Գƽṹ�����ܵĽṹ��ʽΪ��
����ͬ���칹�����ں˴Ź�����������ʾΪ����壬�������Ϊ3��2����״�Ҳ�����֧�����칹�壬�����к���2��-CH3��2��-CH2-����Ϊ�Գƽṹ�����ܵĽṹ��ʽΪ��
CH3CH2COC��CCOCH2CH3��CH3COCH2C��CCH2COCH3��
CH3CH2OC��C-C��COCH2CH3��CH3OCH2C��C-C��CCH2OCH3��
CH3OC��CCH2-CH2C��COCH3��CH3C��COCH2-CH2OC��CCH3��
CH3C��CCH2OOCH2C��CCH3��CH3CH2C��COOC��CCH2CH3��
����8�֣�����ij�칹��L�еĹ����Ŷ�����H2�����ӳɷ�Ӧ����L�Ľṹ��ʽΪ CH3CH2COC��CCOCH2CH3��CH3COCH2C��CCH2COCH3��
�ʴ�Ϊ��8��CH3CH2COC��CCOCH2CH3��CH3COCH2C��CCH2COCH3��
���� ���⿼���л�����ƶ���ϳɡ������Žṹ���л���Ӧ����ʽ��д����������ͬ���칹�����д�ȣ���3����ͬ���칹����дΪ�״��㡢�ѵ㣮

 �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
| A�� | һ����˵������������Ӧ�Ļ�ԭ���ﲻ�ǵ�һ�� | |
| B�� | ��һ�����������������9.75mol•L-1HNO3��Һ��Ӧ�õ���̬������2.24L����μӷ�Ӧ����������ʵ���Ϊ0.1mol�� | |
| C�� | �����Ũ��Խ���仹ԭ�����м�̬Խ�ߵijɷ�Խ�� | |
| D�� | ������Ũ��Ϊ9.75mol•L-1ʱ��ԭ������NO��NO2��N2O���������ʵ���֮��Ϊ5��3��1 |
| A�� | 1��2��3 | B�� | 3��2��1 | C�� | 2��3��6 | D�� | 6��3��2 |
 ��������ˮ���д����Ƿ�ֹˮ����Ⱦ������ˮ�ʵ���Ҫ��ʩ��������������������������Һ������Ⱦ����������ӣ�ͬʱ�õ������ԭ����ͼ��ʾ��ͼ�С�HA����ʾ������ӣ�A-��ʾ��������ӣ���������������ȷ���ǣ�������
��������ˮ���д����Ƿ�ֹˮ����Ⱦ������ˮ�ʵ���Ҫ��ʩ��������������������������Һ������Ⱦ����������ӣ�ͬʱ�õ������ԭ����ͼ��ʾ��ͼ�С�HA����ʾ������ӣ�A-��ʾ��������ӣ���������������ȷ���ǣ�������| A�� | ��������Һ��pH��С | |
| B�� | �����ĵ缫��ӦʽΪ2H2O-4e-�T4H++O2�� | |
| C�� | ͨ��һ��ʱ���Ũ����ˮ�������٣�����������ҺŨ������ | |
| D�� | �����������ӽ���Ĥ����λ�ã�����ͼ���Դﵽ��ͬ��Ч�� |
| A�� | 46g NO2��N2O4������Ȼ�ϣ����û����������ԭ����ΪNA | |
| B�� | ��״���£���2NA�������ļ�ȩ������ռ�����ԼΪ22.4 L | |
| C�� | 1mol Fe ��һ���������ᷴӦ��ת�Ƶĵ�����Ϊ0.2NA��0.3NA | |
| D�� | 1mol N2��3mol H2��ϣ����ܱ������г�ַ�Ӧ�������ڵ�N-H�������ܵ���5NA |
��ȡ��Һ��������ϡ���ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬����Һ������������䣮
����ȡ��Һ����BaCl2��Һ���а�ɫ�������ɣ�
��������ʵ�飬�����Ʋ���ȷ���ǣ�������
| A�� | ����ڵİ�ɫ��������ΪBaSO4��BaCO3 | |
| B�� | ������Һ��ɫ��Ӧ����ɫ�������Һһ����6��������� | |
| C�� | ������п���ȷ��Fe2+��NO3-�Ĵ��ڣ�����ȷ���������ӵĴ��� | |
| D�� | ��Һ������ȷ��Al3+�Ĵ������ |
 ������Ԫ��X��Y��Z��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������Yԭ�ӵ��������������ڲ������������������˵������ȷ���ǣ�������
������Ԫ��X��Y��Z��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������Yԭ�ӵ��������������ڲ������������������˵������ȷ���ǣ�������| A�� | YԪ������Ԫ�ؿ��γ�H2Y2�͵����ӻ����� | |
| B�� | ZԪ�صĵ����а뵼�����ԣ�ԭ�Ӱ뾶��Z��X | |
| C�� | ����������Ӧˮ��������ԣ�HXO3��H3WO4 | |
| D�� | Ԫ��Z��W��������۷ֱ���������������� |

 ����ش��������⣺
����ش��������⣺ ��
�� ��д�ṹ��ʽ��һ�ּ��ɣ���
��д�ṹ��ʽ��һ�ּ��ɣ��� �ĺϳ�·������ͼ�����Լ���ѡ����
�ĺϳ�·������ͼ�����Լ���ѡ���� ��
��