��Ŀ����
2����ʵ�������������ƹ�������100mL 2mol•L-1��NaOH��Һ���ش��������⣺��1�����в�����˳���ǣ�ÿ����ѡһ�Σ�ABGECDF��
A������ B���ܽ� C��ϴ�� D������ E��ת�� F��ҡ�� G����ȴ
��2��������ƿ������������ˮ��������Һ��Ũ�Ƚ���Ӱ�죮���ƫ����ƫС������Ӱ�족��
��3�����ƹ�������IJ����������ձ�����Ͳ����ͷ�ιܡ�����ƿ�Ͳ�������
��4��д��Na2O2��ˮ��Ӧ�Ļ�ѧ����ʽ2Na2O2+2H2O=4NaOH+O2����
��5����ˮ��Na2O2��Ӧ����H2O2��д���÷�Ӧ�Ļ�ѧ����ʽNa2O2+2H2O=2NaOH+H2O2��
��6��H2O2��ʹ����KMnO4��Һ��ɫ����֪������صIJ���ΪMn2+����ʱH2O2�����˻�ԭ�ԣ����������ԭ�������÷�Ӧ�����ӷ���ʽΪ2MnO4-+5H2O2+6H+=2Mn2++5O2��+8H2O��
���� ��1����������һ�����ʵ���Ũ�ȵ���Һ�����������
��2������ƿ������������ˮ�����������Ƶ���Һ��������ʵ����ʵ���û��Ӱ�죻
��3����������������ʹ�õIJ����������ش�
��4���������ƿ��Ժ�ˮ֮�䷢����Ӧ�����������ƺ�������
��5���������ƿ��Ժ�ˮ֮�䷢�����ֽⷴӦ�õ��������ƺ�˫��ˮ��
��6����Ӧ�У����ϼ�����Ԫ�����ڵķ�Ӧ���ǻ�ԭ����������ؾ��������ԣ��ܽ���ԭ�Ե�����˫��ˮ������
��� �⣺��1������100mL 2mol•L-1��NaOH��Һ����Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ���ȡ����ݡ�ҡ�ȵȣ�������ȷ������˳��Ϊ��ABGECDF��
�ʴ�Ϊ��ABGECDF��
��2��������ƿ������������ˮ�����ڶ���ʱ����Ҫ��������ˮ�����Զ�������Һ��Ũ����Ӱ�죬
�ʴ�Ϊ����Ӱ�죻
��3��������ʹ�õIJ����������ձ�������������ͷ�ιܡ�����ƿ��
�ʴ�Ϊ����������
��4���������ƿ��Ժ�ˮ֮�䷢����Ӧ�����������ƺ�����������2Na2O2+2H2O=4NaOH+O2�����ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
��5���������ƿ��Ժ�ˮ֮�䷢�����ֽⷴӦ�õ��������ƺ�˫��ˮ������Na2O2+2H2O=2NaOH+H2O2���ʴ�Ϊ��Na2O2+2H2O=2NaOH+H2O2��
��6��H2O2��ʹ����KMnO4��Һ��ɫ����֪������صIJ���ΪMn2+��Mn�Ļ��ϼ۽����ǣ����Ը����������������˫��ˮ����Ԫ�صĻ��ϼ����ߣ�����˫��ˮ�ǻ�ԭ�������߷����ķ�ӦΪ��2MnO4-+5 H2O2+6H+=2Mn 2++5O2��+8H2O���ʴ�Ϊ����ԭ��2MnO4-+5H2O2+6H+=2Mn2++5O2��+8H2O��
���� ���⿼����һ�����ʵ���Ũ�ȵ���Һ���ơ����ʵ������Լ���Ӧ����ʽ����д��֪ʶ����Ŀ�Ѷ��еȣ�ע����������һ��Ũ����Һ�ķ�������ȷ������ԭ��Ӧ����д�����ǹؼ���

 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
���ֺ�����������ˮ�е��ܽ������±���
| ���� | V2O5 | NH4VO3 | VOSO4 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |
��1����Ӧ��������Һ�г�H+֮�����������VO2+��Al3+��
��2����Ӧ�ڼ�����˳��Ĺ�����Ҫ�ɷ���Al��OH��3��д��ѧʽ����
��3����Ӧ�ܵ����ӷ���ʽΪVO3-+NH4+=NH4VO3����
��4��25�桢101 kPaʱ��4Al��s��+3O2��g���T2Al2O3��s����H1=-a kJ/mol
4V��s��+5O2��g���T2V2O5��s����H2=-b kJ/mol
��V2O5�������ȷ�Ӧұ�����������Ȼ�ѧ����ʽ��10Al��s��+3V2O5��s��=5Al2O3��s��+6V��s����H=-$\frac{5a-3b}{2}$kJ/mol��
��5����Һ����أ���ͼ2��ʾ�����й�����Ӧ��������г�ǰ�����õ���и�Ĥֻ����H+ͨ������طŵ�ʱ�����ĵ缫��ӦʽΪV2+-e-=V3+����س��ʱ�����ĵ缫��Ӧʽ��VO2+-e-+H2O=VO2++2H+��
��6���������ữ��H2C2O4��Һ�ζ���VO2��2SO4��Һ���Բⶨ��Ӧ�ٺ���Һ�еĺ���������Ӧ�����ӷ���ʽΪ��2VO2++H2C2O4+2H+�T2VO2++2CO2��+2H2O��ȡ25.00mL 0.1000 mol/L
H2C2O4����Һ����ƿ�У�����ָʾ����������Һʢ���ڵζ����У��ζ����յ�ʱ���Ĵ���Һ24.0mL���ɴ˿�֪���ã�VO2��2SO4��Һ�з��ĺ���Ϊ10.6g/L��
�ٽ�X��������ˮ�У��õ�������Y����ҺZ��
��ȡ����Y��������Ũ���ᣬ���ȣ���������ɫ���壬����������ɫ�����
��ȡ������Һ����������KSCN��Һ����Һ�ʺ�ɫ��
�ܽ�Z��Һ�е�����ɫʯ����ֽ�ϣ���ֽ��죬ȡ������Һ�������ữ���Ȼ�����Һ���а�ɫ�������ɣ����ϲ���Һ�еμ���������Һ���а�ɫ������
��������ʵ���������н�����ȷ���ǣ�������
| A�� | X��һ��������FeO | |
| B�� | Y��һ������MnO2��Fe2O3 | |
| C�� | Z��Һ��һ������Na2SO4������ȷ���Ƿ���AlCl3 | |
| D�� | ������Y��һ������Fe��CuO |
| A�� | Zn | B�� | Fe | C�� | Mg | D�� | Al |
| ѡ�� | ʵ����� | ʵ������ | ���ͻ���� |
| A | ��ʢ��ij��Һ���Թ��еμ�NaOH��Һ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ� | ��ֽδ���� | ����Һ�в���NH4+ |
| B | �ø��������Һ������ϩ�л��е�SO2 | ���������Һ��ɫ | ��ϩ�л���SO2 |
| C | ���Ȼ�����Һ��ͨ��CO2 | �õ���ɫ���� | ������BaCO3 |
| D | ����KI��Һ�е�����ˮ����ͨ��SO2 | �ȱ�������ɫ | SO2�л�ԭ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��FeCl3��Һ��ʴͭ��·�壺Cu+Fe3+�TCu2++Fe2+ | |
| B�� | Na2O2��H2O��Ӧ����O2��2Na2O2+2H2O�T4Na++4OH-+O2�� | |
| C�� | ��������ϡ���2Fe+6H+�T2Fe3++3H2�� | |
| D�� | ���Ȼ�����Һ�м�������İ�ˮ��Al3++4NH3•H2O�TAlO2-+4NH4++2H2O |

 +NaOH��
+NaOH�� +CH3OH��
+CH3OH�� ���������ͨ����ȥ��Ӧ���ɻ�����I�Ļ�ѧ����ʽΪ
���������ͨ����ȥ��Ӧ���ɻ�����I�Ļ�ѧ����ʽΪ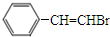 +NaOH$��_{��}^{��}$
+NaOH$��_{��}^{��}$ +NaBr+H2O��ע����Ӧ��������
+NaBr+H2O��ע����Ӧ��������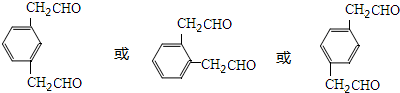
 ����Ľṹ��ʽΪ
����Ľṹ��ʽΪ
 ����HCHO������̼ԭ�Ӳ�ȡsp2�ӻ��ķ����Т٢ۢܣ������ʱ�ţ���HCHO���ӵ����幹��Ϊƽ�������Σ�
����HCHO������̼ԭ�Ӳ�ȡsp2�ӻ��ķ����Т٢ۢܣ������ʱ�ţ���HCHO���ӵ����幹��Ϊƽ�������Σ�