��Ŀ����
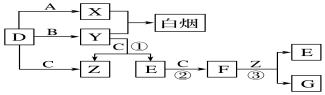
3������A����ͼ��ʾ�Ĺ���ת��Ϊ������D����֪DΪǿ�ᣬ��ش��������⣺A$\stackrel{O_{2}}{��}$B$\stackrel{O_{2}}{��}$C$\stackrel{H_{2}O}{��}$D
��1����A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ���壮
��D�Ļ�ѧʽ��H2SO4��
���ڹ�ҵ������B����Ĵ����ŷű���ˮ���պ��γ����������Ⱦ�˻�����
��д����B��������C���ʵķ�Ӧ����ʽ2SO2+O2$?_{��}^{����}$2SO3��
��2����A�ڳ�����Ϊ���壬��ʹʪ��ĺ�ɫʯ����ֽ��죬C�Ǻ���ɫ�����壮
��A��C�Ļ�ѧʽ�ֱ��ǣ�ANH3��CNO2��
��д��ʵ������ȡ����A�ķ�Ӧ����ʽN2+3H2$?_{����}^{���¸�ѹ}$2NH3��
����д����A����B�ķ�Ӧ����ʽ�����������ת�Ƶķ������Ŀ
 ��
��
���� �ǽ�������A�ܷ������������������յú�����DΪǿ�ᣬ��ѧ����͵�Ԫ�ػ��������ת����ϵ��
��1��A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ���壬��BΪSO2������ת����ϵ��֪AΪSԪ�ء�CΪSO3��DΪH2SO4��
��2����A�ڳ�����Ϊ��̬�������ʹʪ��ĺ�ɫʯ����ֽ��죬��AΪNH3��C�Ǻ���ɫ�����壬��CΪNO2�����ת����ϵ��֪BΪNO��DΪHNO3���ݴ˽��н��
��� �⣺�ǽ�������A�ܷ������������������յú�����DΪǿ�ᣬ��ѧ����͵�Ԫ�ػ��������ת����ϵ��
��1��A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ���壬��AΪSԪ�أ�BΪSO2��CΪSO3��DΪH2SO4��
�ٸ��ݷ���Ϣ��֪��DΪ���ᣬ�仯ѧʽΪ��H2SO4��
�ʴ�Ϊ��H2SO4��
���ڹ�ҵ������SO2����Ĵ����ŷű���ˮ���պ��γ����������Ⱦ�˻�����
�ʴ�Ϊ�����ꣻ
��B��C��Ӧ�Ƕ�������Ĵ�������Ӧ����Ӧ�Ļ�ѧ����ʽ��2SO2+O2$?_{��}^{����}$2SO3��
�ʴ�Ϊ��2SO2+O2$?_{��}^{����}$2SO3��
��2����A�ڳ�����Ϊ��̬�����C�Ǻ���ɫ�����壬��AӦΪNH3��BΪNO��CΪNO2��DΪHNO3��
�������Ϸ�����֪A��C�Ļ�ѧʽ�ֱ���NH3��NO2��
�ʴ�Ϊ��NH3��NO2��
�ڹ�ҵ���õ����������ڴ������������¸��¸�ѹ�ϳɰ�������Ӧ�Ļ�ѧ����ʽΪ��N2+3H2$?_{����}^{���¸�ѹ}$2NH3��
�ʴ�Ϊ��N2+3H2$?_{����}^{���¸�ѹ}$2NH3��
��AΪNH3��BΪNO����Ӧ����ʽΪ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O����Ӧ��OԪ�صõ��ӣ����ϼ۽��ͣ�NԪ��ʧ���ӣ����ϼ����ߣ�ת�Ƶ�����ĿΪ20������ת�Ƶķ������Ŀ�ɱ�ʾΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼��������ƶϣ���Ŀ�Ѷ��еȣ��������ʵ���ɫ�Լ���������������ӦΪͻ�ƿڽ����ƶϣ�ע���������ճ���Ԫ�ؼ��仯�������ʣ�����������ѧ���ķ���������������������

 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�| A�� | 1mol A+2mol B+1mol C | |
| B�� | 0.6mol C+0.6mol D+0.2mol B+0.3mol A | |
| C�� | 0.6mol A+0.2mol D+0.1mol C | |
| D�� | 0.25mol A+0.5mol B+0.1mol C |
| A�� | ${\;}_{23}^{51}$V��${\;}_{23}^{50}$V��Ϊͬλ�� | |
| B�� | ${\;}_{23}^{51}$��${\;}_{23}^{50}$V����������ͬ | |
| C�� | ${\;}_{23}^{51}$V��${\;}_{23}^{50}$V��ͬһ�ֺ��� | |
| D�� | ${\;}_{23}^{51}$V��${\;}_{23}^{50}$V�ĺ������������������Ϊ23 |
| A�� | ���ɰ�ɫ���������� | B�� | �����ɰ�ɫ���� | ||
| C�� | �������ݲ��� | D�� | �ޱ仯 |
| ѡ�� | ���� | ��; |
| A | �ƺͼصĺϽ��ܵ��� | ԭ�ӷ�Ӧ�ѵĵ��ȼ� |
| B | �����������Ư���� | Ư��ֽ�� |
| C | þȼ��ʱ����ҫ�۵�ǿ�� | ���������� |
| D | Al��OH��3�����ֽ� | ���ϵ���ȼ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |

 C(g)��D(g)�Ѵﵽƽ��״̬���ǣ� ��
C(g)��D(g)�Ѵﵽƽ��״̬���ǣ� ��