��Ŀ����
11������A�ǵؿ��к������Ľ���������A���Բ�����ͼ�仯�����ֲ��뷴Ӧ�����ʺͷ�Ӧ����ʡ�ԣ���
��֪��
����A��¶�ڿ�����ʱ��Ѹ������C��D��һ�ֵ���ɫ�Ļ����G�����壻H�������Σ�
��1����д���������ʵĻ�ѧʽ��D��Na2O2 F��NaAlO2 I��Al��OH��3
��2����д����������G�����ӷ���ʽ��2Al+2OH-+2H2O=2AlO2-+3H2��H�백ˮ��Ӧ����I�����ӷ���ʽ��Al3++3NH3•H2O=Al��OH��3��+3NH4+
��3�����ѽ���A��Ƭ�ڿ����м���ʱ���۲쵽�������ǽ���A�ۻ��������䣬ԭ�������������۵�Զ������������ס���ۻ�����ʹ�䲻���䣮
��4��ijͬѧ������ý���A��ŨHNO3�ڳ����·�Ӧֱ�ӵõ���H�ķ�������ͬѧ�ķ����Ƿ��ܴﵽʵ��Ŀ�ģ����ǻ��ԭ���dz���������Ũ����ۻ���
���� ����A�ǵؿ��к������Ľ�������AΪ����Al��Al�ڿ�����Ѹ�ٱ�����������������CΪAl2O3��H�������Σ���HΪAl��NO3��3��D��һ�ֵ���ɫ�Ļ������DΪNa2O2��E�ܹ����������Ӧ��������G����EΪNaOH��GΪH2��FΪNaAlO2��Al��NO3��3��NaAlO2���ܹ�ת����I����IΪAl��OH��3���ݴ˽��н��
��� �⣺����A�ǵؿ��к������Ľ�������AΪ����Al��Al�ڿ�����Ѹ�ٱ�����������������CΪAl2O3��H�������Σ���HΪAl��NO3��3��D��һ�ֵ���ɫ�Ļ������DΪNa2O2��E�ܹ����������Ӧ��������G����EΪNaOH��GΪH2��FΪNaAlO2��Al��NO3��3��NaAlO2���ܹ�ת����I����IΪAl��OH��3��
��1�����ݷ�����֪��DΪNa2O2��FΪ NaAlO2��IΪAl��OH��3��
�ʴ�Ϊ��Na2O2��NaAlO2��Al��OH��3��
��2������GΪ������Al������������Һ��Ӧ����ƫ�����ƺ���������Ӧ�����ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2����
H�백ˮ��Ӧ����I�����ӷ���ʽΪ��Al3++3NH3•H2O=Al��OH��3��+3NH4+��
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����Al3++3NH3•H2O=Al��OH��3��+3NH4+��
��3���������������۵�Զ��������������Ƭ�����ɵ�����������ס���ۻ�����ʹ�䲻���䣬
�ʴ�Ϊ�����������۵�Զ������������ס���ۻ�����ʹ�䲻���䣻
��4�������½�������Ũ���ᷢ���ۻ�������ֹ�˷�Ӧ�ļ������У������²���������Ũ������ȡ��������
�ʴ�Ϊ������������Ũ����ۻ���
���� ���⿼�����ƶϣ���Ŀ�Ѷ��еȣ���ȷ�ƶϸ���������Ϊ���ؼ���ע���������ճ���Ԫ�ؼ��仯�������ʣ�����������ѧ���ķ���������������������

| A�� | һ����Fe2+��Cu2+ | B�� | һ����Fe2+��Cu2+��������Fe3+ | ||
| C�� | һ����Fe2+��������Cu2+ | D�� | ֻ��Fe2+ |
| A�� | Ba2+��Cl-��SO42-��K+ | B�� | Mg2+��SO42-��Na+��Cl- | ||
| C�� | H+��CO32-��Al3+��Cl- | D�� | K+��S O32-��NO3-��H+ |
 2016��12�£��й��������е������������ص������������������ӱ������ϵȵصĿ�����Ⱦ��Ϊ6��������Ⱦ�������ض���Ⱦ������β����ȼú����������ȡů�ŷŵ�CO2�ȶ��������γɵ�ԭ��
2016��12�£��й��������е������������ص������������������ӱ������ϵȵصĿ�����Ⱦ��Ϊ6��������Ⱦ�������ض���Ⱦ������β����ȼú����������ȡů�ŷŵ�CO2�ȶ��������γɵ�ԭ����1������β����������Ҫԭ��Ϊ��2NO��g��+2CO��g��$\stackrel{����}{?}$ N2��g��+2CO2��g����H��0����һ���¶��£���һ����̶����ܱ������г���һ������NO��CO��t1ʱ�̴ﵽƽ��״̬��
�����жϸ÷�Ӧ�ﵽƽ��״̬�ı�־��CD��
A���ڵ�λʱ��������1molCO2��ͬʱ������1molCO B�����������ܶȲ��ٸı�
C����������ƽ����Է����������ٸı� D����������ѹǿ���ٱ仯
����t2ʱ�̣����������ݻ�Ѹ������ԭ����2�����������������������£�t3ʱ�̴ﵽ�µ�ƽ��״̬��֮���ٸı�����������ͼ�в��仭����t2��t4ʱ������Ӧ������ʱ��ı仯���ߣ�
����Ҫͬʱ��߸÷�Ӧ�����ʺ�NO��ת���ʣ���ȡ�Ĵ�ʩ������ѹǿ���������г���CO���壮����д1����
��2���ı�ú�����÷�ʽ�ɼ��ٻ�����Ⱦ��ͨ���ɽ�ˮ����ͨ�����ȵ�̼�õ�ˮú�����䷴ӦΪC��s��+H2O��g��?CO��g��+H2��g����HZZ=+131.3kJ•mol-1
�ٸ÷�Ӧ�ڸ��������Է����У�����¡��������¡��������¶ȡ�����
��ú���������в������к�����H2S����������Na2CO3��Һ���գ��÷�Ӧ�����ӷ���ʽΪCO32-+H2S=HCO3-+HS-������֪��H2S��Ka1=9.1��10-8��Ka2=1.1��10-12��H2CO3��Ka1=4.3��10-7��Ka2=5.6��10-11����
��3����֪��Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g�����ֽ���ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������н��з�Ӧ���õ�������������
| ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
| 2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | d | t |
������900��ʱ������һ��ʵ�飬�ڴ������м���10molCO��5molH2O��2molCO2��5molH2�����ʱV����V���������������������=������
| ѡ�� | ʵ����ʵ | ���� |
| A | H2����Cl2��ȼ�� | ȼ�ղ�һ���������μ� |
| B | �����ھƾ��ƻ����ϼ����ۻ��������� | �������۵������ |
| C | �ƿ�Ͷ��ˮ�У�Ѹ���۳�����ɫС�� | �Ƶ��ܶȱ�ˮС |
| D | ij��Һ�м��������ữ��AgNO3��Һ��������ɫ���� | ����Һ�к���Cl- |
| A�� | A | B�� | B | C�� | C | D�� | D |
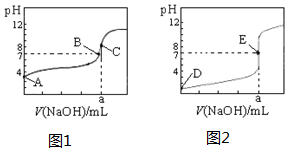 ��ͼΪ��������0.1000mol•L-1NaOH��Һ�ζ�20.00mL 0.1000mol•L-1�����20.00mL 0��.1000mol•L-1��������ߣ�����HA��ʾ�ᣬ�����жϺ�˵������ȷ���ǣ�������
��ͼΪ��������0.1000mol•L-1NaOH��Һ�ζ�20.00mL 0.1000mol•L-1�����20.00mL 0��.1000mol•L-1��������ߣ�����HA��ʾ�ᣬ�����жϺ�˵������ȷ���ǣ�������| A�� | ͼ2�ǵζ���������� | |
| B�� | ͼ1�ζ�ʱӦ��ѡ���̪��ָʾ�� | |
| C�� | B��ʱ����Ӧ������Һ�����V��NaOH����V��HA�� | |
| D�� | ��0 mL��V��NaOH����20.00 mLʱ����Ӧ��Һ�и�����Ũ�ȴ�С˳��һ����Ϊc��A-����c��Na+����c��H+����c��OH-�� |
| A�� |  ���������ζ | B�� |  ��Ũ�������CO2 | ||
| C�� |  ������ƿ��ת��Һ�� | D�� |  �ú�ˮ��ȡ��ˮ |
