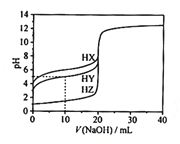
ЬтФПФкШн
ЁОЬтФПЁПвЛжжRuТчКЯЮягыg-C3N4ИДКЯЙтДпЛЏМСНЋCOЃЌЛЙдЮЊHCOOHЕФдРэЭМШчЭМЁЃ

(1)ЛљЬЌЬМдзгЕФМлЕчзгХХВМЭМЮЊ___________ЁЃ
(2)1molHCOOHжаКЌгаЕФІвМќЪ§ФПЮЊ___________ЃЌHCOOHЕФЗаЕуБШCO2ИпЕФдвђЮЊ___________ЁЃ
(3)RuТчКЯЮяжаЕкЖўжмЦкдЊЫиЕФЕквЛЕчРыФмгЩДѓЕНаЁЕФЫГађЮЊ___________ЁЃ
(4)RuТчКЯЮяжагыRuХфЮЛЕФдзггаNЁЂ___________ЁЃ
(5)вЛРрЪЏФЋЕФОлКЯЮяАыЕМЬхg-C3N4ЃЌЦфЕЅВуЦНУцНсЙЙШчЭМ1ЃЌОЇАћНсЙЙШчЭМ2ЁЃ

Ђйg-C3N4жаЕЊдзгЕФдгЛЏРраЭЪЧ______________ЁЃ
ЂкИљОнЭМ2ЃЌдкЭМ1жагУЦНааЫФБпаЮЛГівЛИізюаЁжиИДЕЅдЊЁЃ______________
ЂлвбжЊИУОЇАћЕФЬхЛ§ЮЊVcm3ЃЌжаМфВудзгОљдкОЇАћФкВПЁЃЩшАЂЗќМгЕТТоГЃЪ§ЕФжЕЮЊNAЃЌдђg-C3N4ЕФУмЖШЮЊ______________g.cm-3ЁЃ
ЁОД№АИЁП Лђ
Лђ 4NA HCOOHКЭCO2ЖМЮЊЗжзгОЇЬхЃЌHCOOHЗжзгМфаЮГЩЧтМќ N>O>C ClЃЌC sp2дгЛЏ
4NA HCOOHКЭCO2ЖМЮЊЗжзгОЇЬхЃЌHCOOHЗжзгМфаЮГЩЧтМќ N>O>C ClЃЌC sp2дгЛЏ 
![]()
ЁОНтЮіЁП
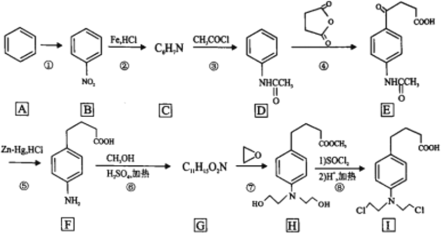
ИљОнФмСПзюЕЭдРэЃЌгЩЛљЬЌЬМдзгЕФМлЕчзгХХВМЪНШЗЖЈЦфМлЕчзгХХВМЭМЃЛHCOOHКЭCO2ЖМЮЊЗжзгОЇЬхЃЌHCOOHЗжзгМфаЮГЩСЫЧтМќЃЌЕМжТЗаЕуЦЋИпЃЛЭЌвЛжмЦкдЊЫиЕФЕквЛЕчРыФмЫцзХдзгађЪ§ЕФдіДѓЖјдіДѓЃЌЧвЭЌвЛжмЦкЕФЕкЂђAдЊЫиЕФЕквЛЕчРыФмДѓгкЕкЂѓAзхЕФЃЌЕкЂѕAзхЕФДѓгкЕкЂіAзхЕФЃЛгЩЭМвЛПЩжЊNаЮГЩШ§ИіЛЏбЇМќЃЌга3ИідгЛЏЙьЕРЃЌдгЛЏЙьЕРМфЕФМаНЧЮЊ120ЁуЁЃ
(1)ИљОнФмСПзюЕЭдРэЃЌЛљЬЌЬМдзгЕФМлЕчзгХХВМЪНЮЊ2s22p2ЃЌЙЪЦфМлЕчзгХХВМЭМЮЊ![]() Лђ
Лђ![]() ЃЛ
ЃЛ
(2)вЛИіЕЅМќОЭЪЧвЛИіІвМќ,вЛИіЫЋМќжаКЌгавЛИіІвМќ,вЛИіІаМќЃЌИљОнМзЫсЗжзгНсЙЙПЩжЊ1ЗжзгМзЫсжаКЌ4ИіІвМќЃЌдђ1molHCOOHжаКЌгаЕФІвМќЪ§ФПЮЊ4NAЃЛHCOOHКЭCO2ЖМЮЊЗжзгОЇЬхЃЌЕЋHCOOHЗжзгМфаЮГЩСЫЧтМќЃЌЧтМќБШЦеЭЈЗжзгМфзїгУСІвЊЧПЃЌЙЪHCOOHЕФЗаЕуБШCO2ИпЃЛ
(3)RuТчКЯЮяжаКЌгаЕФЕкЖўжмЦкдЊЫиЮЊCЁЂNЁЂOЃЌЭЌвЛжмЦкдЊЫиЕФЕквЛЕчРыФмЫцзХдзгађЪ§ЕФдіДѓЖјдіДѓЃЌЧвЭЌвЛжмЦкЕФЕкЂђAдЊЫиЕФЕквЛЕчРыФмДѓгкЕкЂѓAзхЕФЃЌЕкЂѕAзхЕФДѓгкЕкЂіAзхЕФЃЌЙЪЕквЛЕчРыФмгЩДѓЕНаЁЕФЫГађЮЊN>O>CЃЛ
(4)гЩRuТчКЯЮяЕФНсЙЙПЩжЊЃЌЦфжагыRuХфЮЛЕФдзггаNЁЂClЁЂCЃЛ
(5)ЂйгЩЭМвЛПЩжЊNаЮГЩШ§ИіЛЏбЇМќЃЌга3ИідгЛЏЙьЕРЃЌдгЛЏЙьЕРМфЕФМаНЧЮЊ120ЁуЃЌЙЪNдзгЕФдгЛЏРраЭЮЊsp2дгЛЏЃЛ
ЂкИљОнЭМ2ЃЌдкЭМ1жагУЦНааЫФБпаЮЛГівЛИізюаЁжиИДЕЅдЊЮЊ ЃЛ
ЃЛ
ЂлІб=m/V=M/VNA=![]() g.cm-3
g.cm-3

 ДКгъНЬг§ЭЌВНзїЮФЯЕСаД№АИ
ДКгъНЬг§ЭЌВНзїЮФЯЕСаД№АИЁОЬтФПЁПЮвЙњЪЧИіИжЬњДѓЙњЃЌИжЬњВњСПЮЊЪРНчЕквЛЃЌИпТЏСЖЬњЪЧзюЮЊЦеБщЕФСЖЬњЗНЗЈЁЃ
I.вбжЊЗДгІ![]() Fe2O3(s)+CO(g)
Fe2O3(s)+CO(g)![]() Fe(s)+CO2(g)ЁїH=-23.5kJmol-1ЃЌИУЗДгІдк1000ЁцЕФЦНКтГЃЪ§ЕШгк4ЁЃдквЛИіШнЛ§ЮЊ10LЕФУмБеШнЦїжаЃЌ1000ЁцЪБМгШыFeЁЂFe2O3ЁЂCOЁЂCO2Иї1.0molЃЌгІОЙ§l0minКѓДяЕНЦНКтЁЃ
Fe(s)+CO2(g)ЁїH=-23.5kJmol-1ЃЌИУЗДгІдк1000ЁцЕФЦНКтГЃЪ§ЕШгк4ЁЃдквЛИіШнЛ§ЮЊ10LЕФУмБеШнЦїжаЃЌ1000ЁцЪБМгШыFeЁЂFe2O3ЁЂCOЁЂCO2Иї1.0molЃЌгІОЙ§l0minКѓДяЕНЦНКтЁЃ
(1)COЕФЦНКтзЊЛЏТЪ=__ЃЛ
(2)гћЬсИпCOЕФЦНКтзЊЛЏТЪЃЌДйНјFe2O3ЕФзЊЛЏЃЌПЩВЩШЁЕФДыЪЉЪЧ__ЃЛ
a.ЬсИпЗДгІЮТЖШ
b.діДѓЗДгІЬхЯЕЕФбЙЧП
c.бЁШЁКЯЪЪЕФДпЛЏМС
d.МАЪБЮќЪеЛђвЦГіВПЗжCO2
e.ЗлЫщПѓЪЏЃЌЪЙЦфгыЦНКтЛьКЯЦјЬхГфЗжНгДЅ
Ђђ.ИпТЏСЖЬњВњЩњЕФЗЯЦјжаЕФCOПЩНјааЛиЪеЃЌЪЙЦфдквЛЖЈЬѕМўЯТКЭH2ЗДгІжЦБИМзДМЃКCO(g)+2H2(g)![]() CH3OH(g)ЁЃЧыИљОнЭМЪОЛиД№ЯТСаЮЪЬтЃК
CH3OH(g)ЁЃЧыИљОнЭМЪОЛиД№ЯТСаЮЪЬтЃК

(3)ДгЗДгІПЊЪМЕНЦНКтЃЌгУH2ХЈЖШБфЛЏБэЪОЦНОљЗДгІЫйТЪv(H2)=___ЃЛ
(4)вбжЊЧтЦјЕФШМЩеШШ286kJ/molЃЌЧыаДГіМзДМЦјЬхВЛГфЗжШМЩеЕФШШЛЏбЇЗНГЬЪН___ЃЛ
(5)ШєдкЮТЖШКЭШнЦїЯрЭЌЕФШ§ИіУмБеШнЦїжаЃЌАДВЛЭЌЗНЪНЭЖШыЗДгІЮяЃЌВтЕУЗДгІДяЕНЦНКт МЕФгаЙиЪ§ОнШчЯТБэЃК
ШнЦї | ЗДгІЮяЭЖШыЕФСП | ЗДгІЮяЕФ зЊЛЏТЪ | CH3OH ЕФХЈЖШ | ФмСПБфЛЏ(Q1ЁЂQ2ЁЂQ3ОљДѓгк0) |
Мз | 1molCOКЭ2molH2 | ІС1 | c1 | ЗХГіQ1kJШШСП |
вв | 1molCH3OH | ІС2 | c2 | ЮќЪеQ2kJШШСП |
Бћ | 2molCOКЭ4molH2 | ІС3 | c3 | ЗХГіQ3kJШШСП |
дђЯТСаЙиЯЕе§ШЗЕФЪЧ___ЁЃ
A.c1=c2 B.2Q1=Q3 C.2ІС1=ІС3 D.ІС/span>1+ІС2=1
E.ИУЗДгІШєЩњГЩ1molCH3OHЃЌдђЗХГі(Q1+Q2)kJШШСП
Ђѓ.вдМзЭщЮЊШМСЯЕФаТаЭЕчГиЃЌЦфГЩБОДѓДѓЕЭгквдЧтЮЊШМСЯЕФДЋЭГШМСЯЕчГиЃЌФПЧАЕУЕНЙуЗКЕФбаОПЃЌШчЭМЪЧФПЧАбаОПНЯЖрЕФвЛРрЙЬЬхбѕЛЏЮяШМСЯЕчГиЙЄзїдРэЪОвтЭМЁЃЛиД№ЯТСаЮЪЬтЃК

(6)BМЋЩЯЕФЕчМЋЗДгІЪНЮЊ___ЃЛ
(7)ШєгУИУШМСЯЕчГизіЕчдДЃЌгУЪЏФЋзіЕчМЋЕчНт100mL1mol/LЕФСђЫсЭШмвКЃЌЕБСНМЋЪеМЏЕНЕФЦјЬхЬхЛ§ЯрЕШЪБЃЌРэТлЩЯЯћКФЕФМзЭщЕФЬхЛ§ЮЊ__(БъПіЯТ)ЁЃ