��Ŀ����
��Ԫ�ؿ����γɶ��ֻ�����ش��������⣺
��1��N��N�ļ���Ϊ942kJ?mol-1��N-N�����ļ���Ϊ247kJ?mol-1������˵��N2�е� �����ȶ�����С��ҡ�����
��2��N2��O22+��Ϊ�ȵ����壬O22+�ĵ���ʽ�ɱ�ʾΪ ��
��3���£�N2H4�����ӿ���ΪNH3�����е�һ����ԭ�ӱ�-NH2��������ȡ���γɵ���һ�ֵ����⻯�
��NH3���ӵĿռ乹���� ��N2H4���ӽṹʽΪ ��
���¿��������ȼ�ϣ�ȼ��ʱ�����ķ�Ӧ�ǣ�N2O4��l��+2N2H4��l���T3N2��g��+4H2O��g����H=-1038.7kJ?mol-1���÷�Ӧ����4mol N-H�����ѣ����γɵĦм��� mol��
��1��N��N�ļ���Ϊ942kJ?mol-1��N-N�����ļ���Ϊ247kJ?mol-1������˵��N2�е�
��2��N2��O22+��Ϊ�ȵ����壬O22+�ĵ���ʽ�ɱ�ʾΪ
��3���£�N2H4�����ӿ���ΪNH3�����е�һ����ԭ�ӱ�-NH2��������ȡ���γɵ���һ�ֵ����⻯�
��NH3���ӵĿռ乹����
���¿��������ȼ�ϣ�ȼ��ʱ�����ķ�Ӧ�ǣ�N2O4��l��+2N2H4��l���T3N2��g��+4H2O��g����H=-1038.7kJ?mol-1���÷�Ӧ����4mol N-H�����ѣ����γɵĦм���
���㣺�жϼ��ӻ����ӵĹ���,���ȵ���ԭ������Ӧ��
ר�⣺
��������1��N��N�к���2���м���1���Ҽ�����֪N��N����Ϊ942kJ/mol��N-N��������Ϊ247kJ/mol����1���м��ļ���Ϊ
kJ/mol=347.5kJ/mol���Դ��жϣ�
��2���ȵ�����ṹ���ƣ����ݵ������ӵĵ���ʽ��дO22+�ĵ���ʽ��
��3���ٸ��ݼ۲���ӶԻ�������ȷ�����ӿռ乹�ͣ�N2H4��ԭ�Ӽ��Ե�����ʽ���ڣ�
�����÷�ӦN2O4��l��+2N2H4��l���T3N2��g��+4H2O��g����H=-1038.7kJ?mol-1����4molN-H�����ѣ�˵����1mol�²μӷ�Ӧ����1.5mol�������ɣ�ÿ�����������к��������м����ݴ˼����γɵĦм������ʵ�����
| 942-247 |
| 2 |
��2���ȵ�����ṹ���ƣ����ݵ������ӵĵ���ʽ��дO22+�ĵ���ʽ��
��3���ٸ��ݼ۲���ӶԻ�������ȷ�����ӿռ乹�ͣ�N2H4��ԭ�Ӽ��Ե�����ʽ���ڣ�
�����÷�ӦN2O4��l��+2N2H4��l���T3N2��g��+4H2O��g����H=-1038.7kJ?mol-1����4molN-H�����ѣ�˵����1mol�²μӷ�Ӧ����1.5mol�������ɣ�ÿ�����������к��������м����ݴ˼����γɵĦм������ʵ�����
���
�⣺��1��N��N�к���2���м���1���Ҽ�����֪N��N����Ϊ942kJ/mol��N-N��������Ϊ247kJ/mol����1���м��ļ���Ϊ
kJ/mol=347.5kJ/mol����N2�еĦм����ܴ��ڦҼ����ܣ����ȶ���
�ʴ�Ϊ���У�
��2���ȵ�����ṹ���ƣ������к�����������O22+Ҳ��������������O22+�ĵ���ʽ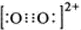 ��
��
�ʴ�Ϊ��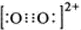 ��
��
��3����NH3�����е�ԭ�Ӻ���3�����ۼ���һ���µ��Ӷԣ����Կռ乹���������ͣ�N2H4������ÿ��Nԭ�Ӻ�����Hԭ���γ��������۵�����Nԭ��֮����ڹ��۵�����������ṹʽΪ ��
��
�ʴ�Ϊ�������Σ� ��
��
�����÷�ӦN2O4��l��+2N2H4��l���T3N2��g��+4H2O��g����H=-1038.7kJ?mol-1����4molN-H�����ѣ�˵����1mol�²μӷ�Ӧ����1.5mol�������ɣ�ÿ�����������к��������м��������γɵĦм���3mol��
�ʴ�Ϊ��3��
| 942-247 |
| 2 |
�ʴ�Ϊ���У�
��2���ȵ�����ṹ���ƣ������к�����������O22+Ҳ��������������O22+�ĵ���ʽ
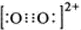 ��
���ʴ�Ϊ��
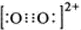 ��
����3����NH3�����е�ԭ�Ӻ���3�����ۼ���һ���µ��Ӷԣ����Կռ乹���������ͣ�N2H4������ÿ��Nԭ�Ӻ�����Hԭ���γ��������۵�����Nԭ��֮����ڹ��۵�����������ṹʽΪ
 ��
���ʴ�Ϊ�������Σ�
 ��
�������÷�ӦN2O4��l��+2N2H4��l���T3N2��g��+4H2O��g����H=-1038.7kJ?mol-1����4molN-H�����ѣ�˵����1mol�²μӷ�Ӧ����1.5mol�������ɣ�ÿ�����������к��������м��������γɵĦм���3mol��
�ʴ�Ϊ��3��
���������⿼����ۺϣ��漰�ȵ����塢���ӿռ乹���жϡ����ܵļ����֪ʶ�㣬���ؿ���ѧ���Ի��������������������ݼ۲���ӶԻ������ۡ��ȵ������ص��֪ʶ�����ѵ���O22+�ĵ���ʽ����д����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
��֪X2��Y2��Z2��W2�������ʵ���������ΪW2��Z2��X2��Y2������������ԭ��Ӧ�ܷ������ǣ�������
| A��2NaW+Z2�T2NaZ+W2 |
| B��2NaX+Z2�T2NaZ+X2 |
| C��2NaW+Y2�T2NaY+W2 |
| D��2NaZ+X2�T2NaX+Z2 |
 �����£���100mL 0.01mol?L-1 HA��Һ����μ���0.02mol?L-1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ���������˵��������ǣ�������
�����£���100mL 0.01mol?L-1 HA��Һ����μ���0.02mol?L-1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ���������˵��������ǣ�������| A�������£�MA��ˮ��Һ��pH=a����ˮ���������c��H+��=1��10-a mol?L-1 |
| B����K�㣬ˮ��Һ�д��ڣ�c��M+��=2c��A-�� |
| C����N�㣬ˮ��Һ�д��ڣ�c��M+��+c��H+��=c��A-��+c��OH-�� |
| D����K�㣬����ʱ��Һ��pH=10����c��MOH��+c��M+��=0.01mol?L-1 |
�����и�����������Һ�У�һ���ܴ���������������ǣ�������
| A����ɫ��Һ��Ca2+��H+��Cl-��HCO3- | ||
B��
| ||
| C��FeCl3��Һ��K+��Na+��SCN-��CO32- | ||
| D����ʹpH��ֽ�ʺ�ɫ����Һ��Na+��NH4+��SO32-��NO3- |
���ڳ�����pH=11�İ�ˮ��Һ������˵������ȷ���ǣ�������
| A����ˮϡ��100����9��pH��11 |
| B����������NH4Cl���壬��Һ��pH���� |
| C����ˮϡ�ͺ�c��OH-��/c��NH3?H2O��ֵ����С |
| D����ȫ�к͵�Ũ�ȵ���������ᣬ�İ�ˮ�������pH=11��NaOH��Һ�� |
���з�Ӧ�л�ѧ������ֻ�漰�м����ѵ��ǣ�������
| A��C2H2��ȼ�� |
| B��C2H4��Cl2�ļӳɷ�Ӧ |
| C��CH4��Cl2��ȡ����Ӧ |
| D��C2H4������KMnO4��Һ���� |



