��Ŀ����
���������������ؾ���K3[Fe��C2O4��3]?3H2O��������Ӱ����ɫӡˢ��ij���⻯ѧ��ȤС��Ϊ�ⶨ�����������ؾ��壨K3[Fe��C2O4��3]?3H2O������Ԫ�غ�������������ʵ�飺
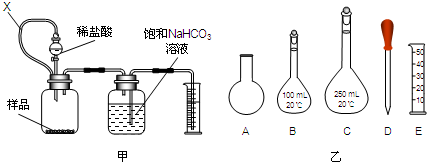
����һ������5.000g�����������ؾ��壬���Ƴ�250ml��Һ��
�������ȡ������Һ25.00ml����ƿ�У���ϡH2SO4�ữ���μ�KMnO4��Һ�������ǡ��ȫ���������ɶ�����̼��ͬʱMnO4-����ԭ��Mn2+����Ӧ�����Һ�м���һ����п�ۣ���������ɫ�պ���ʧ�����ˣ�ϴ�ӣ������˼�ϴ��������Һ�ռ�����ƿ�У���ʱ����Һ�Գ����ԣ�
����������0.010mol/L KMnO4��Һ�ζ������������Һ���յ㣬����KMnO4��ҺV1ml���ζ���MnO4-������ԭ��Mn2+��
�ظ���������������������ζ�����0.010mol/LKMnO4��ҺV2ml��
��1������п�۵�Ŀ���� ��
��2��ʵ�鲽����м�KMnO4��Һ�����ӷ���ʽΪ ���������KMnO4����Һ�������������õ������� ����ѡ�ƫ�͡���ƫ�ߡ������䡱��
��3��ijͬѧ��8.74g��ˮ�����������أ�K3[Fe��C2O4��3]��M=437g/mol����һ�������¼��ȷֽ⣬���ù��������Ϊ5.42g��ͬʱ�õ������ֳɷֵ�������������ɵĻ������ͨ����ʯ������2.2g���о���������֪����Ԫ�ز�������������ʽ���ڣ�����ֻ��K2CO3��KԪ��ֻ���������У���ͨ����Ҫ�ļ��㣬д���÷ֽⷴӦ�Ļ�ѧ����ʽ ��
����һ������5.000g�����������ؾ��壬���Ƴ�250ml��Һ��
�������ȡ������Һ25.00ml����ƿ�У���ϡH2SO4�ữ���μ�KMnO4��Һ�������ǡ��ȫ���������ɶ�����̼��ͬʱMnO4-����ԭ��Mn2+����Ӧ�����Һ�м���һ����п�ۣ���������ɫ�պ���ʧ�����ˣ�ϴ�ӣ������˼�ϴ��������Һ�ռ�����ƿ�У���ʱ����Һ�Գ����ԣ�
����������0.010mol/L KMnO4��Һ�ζ������������Һ���յ㣬����KMnO4��ҺV1ml���ζ���MnO4-������ԭ��Mn2+��
�ظ���������������������ζ�����0.010mol/LKMnO4��ҺV2ml��
��1������п�۵�Ŀ����
��2��ʵ�鲽����м�KMnO4��Һ�����ӷ���ʽΪ
��3��ijͬѧ��8.74g��ˮ�����������أ�K3[Fe��C2O4��3]��M=437g/mol����һ�������¼��ȷֽ⣬���ù��������Ϊ5.42g��ͬʱ�õ������ֳɷֵ�������������ɵĻ������ͨ����ʯ������2.2g���о���������֪����Ԫ�ز�������������ʽ���ڣ�����ֻ��K2CO3��KԪ��ֻ���������У���ͨ����Ҫ�ļ��㣬д���÷ֽⷴӦ�Ļ�ѧ����ʽ
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺
��������1��п������Fe3+��Ӧ������п�۵�Ŀ���ǽ�Fe3+ǡ�û�ԭ��Fe2+��
��2�����������Եĸ�����ط���������ԭ��Ӧ�����ɶ�����̼�������Ӻ�ˮ�������KMnO4����Һ�������㣬���²������ʣ�࣬�ٵζ���������ʱ�������������ط�Ӧ��ʹ�ĵζ������������ĵĸ�����ص����ƫ��
��3���������ɵĻ������ͨ����ʯ������2.2g��������֮һΪC02������n��CO2��=
=0.05mol������ԭ���غ���һ������ֻ��ΪCO��������Ϊ8.74-5.42-2.2=1.12g����n��CO��=
=0.04mol��������Ϣ����������У���Ԫ�ز�������������ʽ���ڣ�����ֻ��K2CO3����ϵ��ӵ�ʧ�غ㣬���Ʋ���Ԫ��ֻ���������������ʵ���ʽ���ڽ��ԭ���غ��������ʵ���֮�ȣ��Ӷ���д����ʽ��
��2�����������Եĸ�����ط���������ԭ��Ӧ�����ɶ�����̼�������Ӻ�ˮ�������KMnO4����Һ�������㣬���²������ʣ�࣬�ٵζ���������ʱ�������������ط�Ӧ��ʹ�ĵζ������������ĵĸ�����ص����ƫ��
��3���������ɵĻ������ͨ����ʯ������2.2g��������֮һΪC02������n��CO2��=
| 2.2 |
| 44 |
| 1.12 |
| 28 |
���
�⣺��1����Ӧ�����Һ�м���һС��п�ۣ���������Һ��ȫ�ɻ�ɫ��Ϊdz��ɫ��˵������п�۵�Ŀ���ǽ�Fe3+ǡ�û�ԭ��Fe2+��
�ʴ�Ϊ����ԭFe3+ΪFe2+��
��2�����������Եĸ�����ط���������ԭ��Ӧ�����ɶ�����̼�������Ӻ�ˮ�����ӷ���ʽΪ��2MnO4-+5H2C2O4+6H+�T10CO2��+2Mn2++8H2O�������KMnO4����Һ�������������²������ʣ�࣬�ٵζ���������ʱ�������������ط�Ӧ��ʹ�ĵζ������������ĵĸ�����ص����ƫ���²ⶨ���������ӵ����ʵ���ƫ����Ԫ�ص�����ƫ����ⶨ����Ԫ�ص���������ƫ�ߣ��ʴ�Ϊ��2MnO4-+5H2C2O4+6H+�T10CO2��+2Mn2++8H2O��ƫ�ߣ�
��3�������ɵĻ������ͨ����ʯ������2.2g��������֮һΪC02������n��CO2��=
=0.05mol������ԭ���غ���һ������ֻ��ΪCO��������Ϊ8.74-5.42-2.2=1.12g����n��CO��=
=0.04mol����������У���Ԫ�ز�������������ʽ���ڣ�����ֻ��K2CO3����ϵ��ӵ�ʧ�غ㣬���Ʋ���Ԫ��ֻ���������������ʵ���ʽ���ڣ���8.74g��ˮ�����������أ�K3[Fe��C2O4��3]��M=437g/mol�������ʵ���Ϊ��
=0.02mol�����Ը��ݼ��غ���n��K2CO3��=0.03mol���������غ���n��FeO��=0.01mol�����غ���n��Fe��=0.01mol�����Է�Ӧ����ʽΪ��2K3[Fe��C2O4��3]�T3K2CO3+Fe+FeO+4CO��+5CO2�����ʴ�Ϊ��2K3[Fe��C2O4��3]�T3K2CO3+Fe+FeO+4CO��+5CO2����
�ʴ�Ϊ����ԭFe3+ΪFe2+��
��2�����������Եĸ�����ط���������ԭ��Ӧ�����ɶ�����̼�������Ӻ�ˮ�����ӷ���ʽΪ��2MnO4-+5H2C2O4+6H+�T10CO2��+2Mn2++8H2O�������KMnO4����Һ�������������²������ʣ�࣬�ٵζ���������ʱ�������������ط�Ӧ��ʹ�ĵζ������������ĵĸ�����ص����ƫ���²ⶨ���������ӵ����ʵ���ƫ����Ԫ�ص�����ƫ����ⶨ����Ԫ�ص���������ƫ�ߣ��ʴ�Ϊ��2MnO4-+5H2C2O4+6H+�T10CO2��+2Mn2++8H2O��ƫ�ߣ�
��3�������ɵĻ������ͨ����ʯ������2.2g��������֮һΪC02������n��CO2��=
| 2.2 |
| 44 |
| 1.12 |
| 28 |
| 8.74 |
| 437 |
������������Ҫ����ʵ��Ļ�������������㣬ע�����ʵ��֪ʶ�Ļ��ۣ�����ʵ�鲽�衢ԭ����ע����������⣮

��ϰ��ϵ�д�
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
�����Ŀ
���з�Ӧ�п��ж�Ϊ���淴Ӧ���ǣ�������
| A��������������ȼ�����Ȼ��⣬�Ȼ������ȷֽ�Ϊ���������� |
| B�������������ڸ��¡���ѹ�����������¿������ɰ�����ͬʱ�����ַֽ�Ϊ���������� |
| C������������û����⣬�����ֿ����û����� |
| D��������ˮ��Ӧ��������ʹ����ᣬ��������������¿ɷֽ�Ϊ��������� |
���ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۼ���������������ȷ���ǣ�������
| Ԫ�ش��� | K | L | M | Q | R | T | N |
| ԭ�Ӱ뾶/nm | 0.186 | 0.160 | 0.143 | 0.102 | 0.089 | 0.074 | 0.152 |
| ��Ҫ���ϼ� | +1 | +2 | +3 | +6��-2 | +2 | -2 | +1 |
| A����RCl2�У���ԭ������������8���ӵ��ȶ��ṹ |
| B��Ԫ��L��N����������Ϊ����ͬһ���� |
| C��K��M��Q��Ԫ������������Ӧ��ˮ��������֮��ɷ�����ѧ��Ӧ |
| D���⻯��е㣺H2Q��H2T |
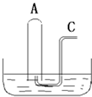 ��ͼ��ʾ����B����װ��500mLˮ���ݻ�Ϊa mL���Թ�A������NO2��NO�Ļ�����壨��״���������Թ�A������B�۵�ˮ�У���ַ�Ӧ���Թ�A��������������Ϊ0.5amL��
��ͼ��ʾ����B����װ��500mLˮ���ݻ�Ϊa mL���Թ�A������NO2��NO�Ļ�����壨��״���������Թ�A������B�۵�ˮ�У���ַ�Ӧ���Թ�A��������������Ϊ0.5amL��