��Ŀ����
��1��ij����������Ԫ�ص�ԭ��M������һ����������Dz㣬����ԭ�ӵ��������� ��д��������Χ�����Ų�ͼ ��
��2��VIA�������Se�����ڻ������г����ֳ���������̬��H2SeO4��H2SeO3���� �� ��ǿ��������H2Se�����Ա�H2S ���ǿ������������
��3������A�Ļ�ѧʽΪNH5����������ԭ�ӵ�����㶼������Ӧϡ������ԭ�ӵ�������Ӳ�ṹ���������й�˵������ȷ����
A��NH5�м������Ӽ����й��ۼ�
B��NH5���ۡ��е����NH3
C��NH5����Ͷ������ˮ�У��ɲ�����������
D��0.1mol NH5���5mol N-H��
��4���������ʾʽд��HF��Һ�д��ڵ�������� ��
��2��VIA�������Se�����ڻ������г����ֳ���������̬��H2SeO4��H2SeO3����
��3������A�Ļ�ѧʽΪNH5����������ԭ�ӵ�����㶼������Ӧϡ������ԭ�ӵ�������Ӳ�ṹ���������й�˵������ȷ����
A��NH5�м������Ӽ����й��ۼ�
B��NH5���ۡ��е����NH3
C��NH5����Ͷ������ˮ�У��ɲ�����������
D��0.1mol NH5���5mol N-H��
��4���������ʾʽд��HF��Һ�д��ڵ��������
���㣺ԭ�Ӻ�������Ų�,Ԫ�������ɵ�����,���ӻ�����Ľṹ����������,���ۼ����γɼ����ۼ�����Ҫ����,�������������
ר�⣺ԭ�������ṹר��,��ѧ���뾧��ṹ
��������1��ij����������Ԫ�ص�ԭ��M������һ����������Dz㣬������ԭ�ӵ����Ų�Ϊ3s1��3s23p3��
��2��H2SeO4�� H2SeO3��Ƚϣ�Se�Ļ��ϼ�Խ�ߣ�����Խǿ���ǽ�����Խǿ��Ԫ�أ�������Ԫ�صĽ������Խǿ�������⻯����ˮ��Һ�о�Խ�ѵ�⣬���Ծ�Խ����
��3��A�Ļ�ѧʽΪNH5����������ԭ�ӵ�����㶼������Ӧϡ������ԭ�ӵ�������Ӳ�ṹ����A�д���笠����Ӻ�H-��Ϊ���ӻ�����Դ������
��4�����γ������ԭ��ΪN��O��F����ԭ��֮�䣬����Ƿ���֮���ǿ���������
��2��H2SeO4�� H2SeO3��Ƚϣ�Se�Ļ��ϼ�Խ�ߣ�����Խǿ���ǽ�����Խǿ��Ԫ�أ�������Ԫ�صĽ������Խǿ�������⻯����ˮ��Һ�о�Խ�ѵ�⣬���Ծ�Խ����
��3��A�Ļ�ѧʽΪNH5����������ԭ�ӵ�����㶼������Ӧϡ������ԭ�ӵ�������Ӳ�ṹ����A�д���笠����Ӻ�H-��Ϊ���ӻ�����Դ������
��4�����γ������ԭ��ΪN��O��F����ԭ��֮�䣬����Ƿ���֮���ǿ���������
���
�⣺��1��ij����������Ԫ�ص�ԭ��M������һ����������Dz㣬������ԭ�ӵ����Ų�Ϊ3s1��3s23p3��
M��Ϊ3s1��ԭ�Ӻ�������Ų�ʽΪ[Ne]3s1��������Ϊ11��ΪNa��ԭ�ӵ���Χ�����Ų�ͼΪ ��
��
M��Ϊ3s23p3��ԭ�Ӻ�������Ų�ʽΪ[Ne]3s23p3��������Ϊ15��ΪP��ԭ�ӵ���Χ�����Ų�ͼ ��
��
�ʴ�Ϊ��11��15�� ��
�� ��
��
��2��ͬһԪ�صĺ������л��ϼ�Խ�ߣ�����Խǿ�����H2SeO4��H2SeO3����ǿ���ǽ�����Խǿ��Ԫ�أ�������Ԫ�صĽ������Խǿ�������⻯����ˮ��Һ�о�Խ�ѵ�⣬���Ծ�Խ�����ǽ�����S��Se������H2Se�����Ա�H2Sǿ��
�ʴ�Ϊ��ǿ��ǿ��
��3��A��NH5�д���笠����Ӻ�H-��Ϊ���ӻ����笠������к�N-H���ۼ�����A��ȷ��
B��NH5Ϊ���Ӿ��壬����Ϊ���Ӿ��壬��NH5���۷е����NH3����B��ȷ��
C��NH5����Ͷ������ˮ�У��ɲ��������������������壬��C��ȷ��
D��0.1mol NH5���0.4mol N-H������D����
�ʴ�Ϊ��ABC��
��4��������ˮ��Һ������HF�����Լ�H2O���ӣ����ڵ��������Ϊ��F-H��F��F-H��O��O-H��F��O-H��O��
�ʴ�Ϊ��F-H��F��F-H��O��O-H��F��O-H��O��
M��Ϊ3s1��ԭ�Ӻ�������Ų�ʽΪ[Ne]3s1��������Ϊ11��ΪNa��ԭ�ӵ���Χ�����Ų�ͼΪ
 ��
��M��Ϊ3s23p3��ԭ�Ӻ�������Ų�ʽΪ[Ne]3s23p3��������Ϊ15��ΪP��ԭ�ӵ���Χ�����Ų�ͼ
 ��
���ʴ�Ϊ��11��15��
 ��
�� ��
����2��ͬһԪ�صĺ������л��ϼ�Խ�ߣ�����Խǿ�����H2SeO4��H2SeO3����ǿ���ǽ�����Խǿ��Ԫ�أ�������Ԫ�صĽ������Խǿ�������⻯����ˮ��Һ�о�Խ�ѵ�⣬���Ծ�Խ�����ǽ�����S��Se������H2Se�����Ա�H2Sǿ��
�ʴ�Ϊ��ǿ��ǿ��
��3��A��NH5�д���笠����Ӻ�H-��Ϊ���ӻ����笠������к�N-H���ۼ�����A��ȷ��
B��NH5Ϊ���Ӿ��壬����Ϊ���Ӿ��壬��NH5���۷е����NH3����B��ȷ��
C��NH5����Ͷ������ˮ�У��ɲ��������������������壬��C��ȷ��
D��0.1mol NH5���0.4mol N-H������D����
�ʴ�Ϊ��ABC��
��4��������ˮ��Һ������HF�����Լ�H2O���ӣ����ڵ��������Ϊ��F-H��F��F-H��O��O-H��F��O-H��O��
�ʴ�Ϊ��F-H��F��F-H��O��O-H��F��O-H��O��
���������⿼����Ԫ�ص����ʵ��ƶϺͻ�ѧ�����������ʵ�Ӱ���Լ�����ı�ʾ�������ۺ��Խ�ǿ��Ϊ��Ƶ���㣬�Ѷ��еȣ�ע������ͬһԪ�صĺ����ᡢ�⻯������ǿ���ıȽϷ�����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�����������ʵ��ó��Ľ�����ȷ���ǣ�������
| A����ij��Һ�м���ϡ���ᣬ����������ͨ�����ʯ��ˮ��ʯ��ˮ����ǣ�����Һһ����̼������Һ |
| B���ò�˿պȡ����ij��Һ������ɫ��Ӧ������ʻ�ɫ������Һһ����������Һ |
| C��Al��Fe��Cu��Ӧ��������������ᷴӦ�����κ�ˮ�����ֽ������������Ϊ���������� |
| D����ij��Һ�еμ���ˮ���ٵμ�KSCN��Һ����Һ��Ѫ��ɫ������Һ�в�һ����Fe2+ |
�����йر�����ȷ���ǣ�������
| A�������ӵĵ����Ų�ʽ��1s22s22p63s23p4 |
B��H2O�ĵ���ʽ��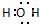 |
C��Nԭ���������ӵĹ����ʾʽ��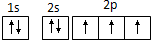 |
D��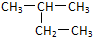 �����ƣ�2-�һ����� �����ƣ�2-�һ����� |
���м���Ԫ�صı���ʽ������ǣ�������
A��F-�ĵ����Ų�ͼ��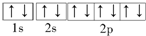 |
B��Na+�Ľṹʾ��ͼ��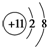 |
| C��Mg2+�ĵ����Ų�ʽ��1s22s22p6 |
| D��Cr�ļ����Ų�ʽ��[Ar]3d44s2 |
����ԭ������ͬ�ĵ��Ӳ�ṹ�����ǣ�������
| A��He |
| B��K+ |
| C��Cl- |
D�� |
���з��ӻ������У�����ԭ�Ӻ��йµ��ӶԵ��ǣ�������
| A��H3O+ |
| B��SiH4 |
| C��PH3 |
| D��SO42- |
����˵����ȷ���ǣ�������
| A��һ���¶��£�ij��Һ��pH��7�������Һ������ |
| B����ˮ�м�������̼���ƹ��彫����ˮ�ĵ��� |
| C��0.02mol?L-1CH3COOH��Һ��0.01mol?L-1NaOH��Һ�������ϣ�����Һ�У�2c��H+��+c��CH3COOH��=2 c��OH-��+c��CH3COO-�� |
| D��Ũ�Ⱦ�Ϊ0.1mol/L��NH4Cl��Һ��NH4HSO4��Һ��ǰ�ߵ�c��NH4+�����ں��� |