��Ŀ����
��13�֣�
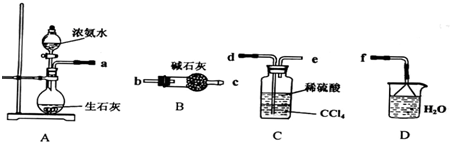
��1��ij��ѧ��ȤС������ϡ�����봿п����ȡ����ʱ���ַ�Ӧ���ʽ�����Ϊ�˼ӿ�÷�Ӧ���ʣ����ǽ����˶���̽����������ǵ�̽���ش��������⣺
�����ǽ��е�����̽���в��ܴﵽʵ��Ŀ�ĵ���____________________����ѡ�
A�����߷�Ӧ�¶� B���ʵ����������Ũ�� C����ˮ D����п������п��
������̽���л����ּ�����������ͭ��ҺҲ�ɼӿ��������������ʡ�����Ϊԭ���ǣ�
____________________________________________________________ ��
����ʵ���г�������ӦZn+H2SO4=ZnSO4+H2������������____________________�������ӷ�Ӧ����ʽ��
�����Ƿ�������Ϊ����ijһ������ҺҲ����������ͭ�����ã�����Ϊ��______________����ѡ�
A��Na2SO4 B��Ag2SO4 C��MgSO4 D��K2SO4
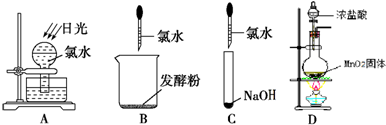
��2����������ѧ���й�ʵ�飬��д���пհס�
����ʵ������ȡ��������ʱ���������Ƭ��������____________________��
��ʯ�����·ֽ�ʵ���У��������Ƭ��Ҫ��____________________���á�
����ʵ������ȡ��������ʱ�����Ƶõ�����������������____________________ ���Դﵽ��ȥ���ʺ��ռ������Ŀ�ġ�
�����һ���Ƚϴ�����̼������ǿ����ʵ��____________________���������ӷ���ʽ��ʾ��
����13�֣���1���� C��2�֣�
�� CuSO4��Zn��Ӧ������ Cu ��Zn�γ�ͭпԭ��أ��ӿ����������������ʣ�2�֣�
Zn + Cu2+ = Zn2+ + Cu��2�֣�
�� B��2�֣�
��2���ٷ�ֹ�������У�1�֣� �ڴ���1�֣� �۱���̼������Һ��1�֣�
��2CH3COOH + CO32-===2CH3COO- + H2O+CO2�����������Ҳ���֣���2�֣�
����

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�