��Ŀ����
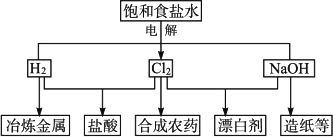
��ˮ�ǻ�ѧԪ�صı��⣬�Ӻ�ˮ�п�����ȡ���������ֻ���ԭ�ϣ���ʳ�Ρ������������塢þ������ȣ������ڹ�ũҵ���������Ź㷺����;������ѧ����֪ʶ�ش��������⣺��1��ij��ѧ��ȤС��ƻ�������������������ȡ��������Һ��
���䷴Ӧԭ��Ϊ���������ӷ���ʽ��ʾ��
������250mL 4.0mol?L-1NaOH��Һ����Ҫ�õ��IJ������������ձ����������⣬�������õ�����
��2����ҵ�Ƶôֹ���������������ʷ�����Ӧ�����յõ����裮��д����ҵ��ȡ�ֹ�Ļ�ѧ����ʽ��
��3������þ����Ϊ����������������þ��𣬲�����CO2�������ԭ����û�ѧ����ʽ��ʾΪ
��4������Na2CO3�����л�������NaHCO3���ʣ���ȥ���ʵķ�����
����NaHCO3��Һ�л�������Na2CO3���ʣ���ȥ���ʵķ�����
��������1��������������������ȡ��������Һ�õ�����������Һ��
������������Һ�IJ�������жϣ�������ȡ����������Һ�����ձ����ܽ���ȴ���ز�����ע��250ml����ƿ������ý�ͷ�ιܶ��ݣ�
��2����ҵ�Ʊ��ֹ������ö��������̼���¼��ȷ�Ӧ�õ��ֹ裻
��3��þ�ڶ�����̼������ȼ����������þ��̼��
��4����Na2CO3�����л�������NaHCO3���ʣ�����̼���������ȷֽ���ӣ�
��NaHCO3��Һ�л�������Na2CO3���ʣ�����ͨ�������̼�����̼���Ʒ�Ӧ����̼�����Ƴ�ȥ��
������������Һ�IJ�������жϣ�������ȡ����������Һ�����ձ����ܽ���ȴ���ز�����ע��250ml����ƿ������ý�ͷ�ιܶ��ݣ�
��2����ҵ�Ʊ��ֹ������ö��������̼���¼��ȷ�Ӧ�õ��ֹ裻
��3��þ�ڶ�����̼������ȼ����������þ��̼��
��4����Na2CO3�����л�������NaHCO3���ʣ�����̼���������ȷֽ���ӣ�
��NaHCO3��Һ�л�������Na2CO3���ʣ�����ͨ�������̼�����̼���Ʒ�Ӧ����̼�����Ƴ�ȥ��
����⣺��1��������������������ȡ��������Һ�õ�����������Һ����Ӧ�����ӷ���ʽΪ��Cl2+2OH-�TCl-+ClO-+H2O��
�ʴ�Ϊ��Cl2+2OH-�TCl-+ClO-+H2O��
��������Һ���������Ӧ�ȼ�����������������Һ���������Ͳ��ȡ����������Һ�����ձ����ܽ���ȴ���ز�����ע��250ml����ƿ������ý�ͷ�ιܶ�����Ҫ�õ��IJ������������ձ����������⣬�������õ����ǣ�250mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��2����ҵ�Ʊ��ֹ������ö��������̼���¼��ȷ�Ӧ�õ��ֹ裬��Ӧ�Ļ�ѧ����ʽΪ��SiO2+2C
Si+2CO��
�ʴ�Ϊ��SiO2+2C
Si+2CO��
��3��þ�ڶ�����̼������ȼ����������þ��̼����Ӧ�Ļ�ѧ����ʽΪ��2Mg+CO2
2MgO+C��
�ʴ�Ϊ��2Mg+CO2
2MgO+C��
��4����Na2CO3�����л�������NaHCO3���ʣ�����̼���������ȷֽ���ӣ�2NaHCO3
Na2CO3+CO2��+H2O��
�ʴ�Ϊ�����ȣ�
��NaHCO3��Һ�л�������Na2CO3���ʣ�����ͨ�������̼�����̼���Ʒ�Ӧ����̼�����Ƴ�ȥ��Na2CO3+CO2+H2O=2NaHCO3��
�ʴ�Ϊ��ͨ�����CO2��
�ʴ�Ϊ��Cl2+2OH-�TCl-+ClO-+H2O��
��������Һ���������Ӧ�ȼ�����������������Һ���������Ͳ��ȡ����������Һ�����ձ����ܽ���ȴ���ز�����ע��250ml����ƿ������ý�ͷ�ιܶ�����Ҫ�õ��IJ������������ձ����������⣬�������õ����ǣ�250mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��2����ҵ�Ʊ��ֹ������ö��������̼���¼��ȷ�Ӧ�õ��ֹ裬��Ӧ�Ļ�ѧ����ʽΪ��SiO2+2C
| ||
�ʴ�Ϊ��SiO2+2C
| ||
��3��þ�ڶ�����̼������ȼ����������þ��̼����Ӧ�Ļ�ѧ����ʽΪ��2Mg+CO2
| ||
�ʴ�Ϊ��2Mg+CO2
| ||
��4����Na2CO3�����л�������NaHCO3���ʣ�����̼���������ȷֽ���ӣ�2NaHCO3
| ||
�ʴ�Ϊ�����ȣ�
��NaHCO3��Һ�л�������Na2CO3���ʣ�����ͨ�������̼�����̼���Ʒ�Ӧ����̼�����Ƴ�ȥ��Na2CO3+CO2+H2O=2NaHCO3��
�ʴ�Ϊ��ͨ�����CO2��
���������⿼����ʵ��������������գ��������ʵķ���Ӧ�ã����ջ����ǹؼ�����Ŀ�ϼ�

��ϰ��ϵ�д�
�����Ŀ