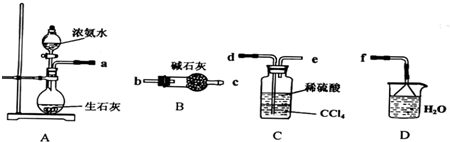
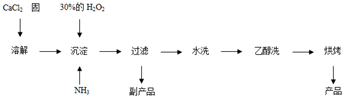
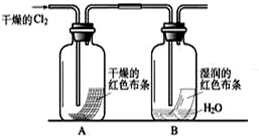
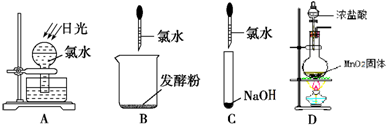
��Ŀ����
��1��ij��ѧ��ȤС��ԡ�ũ��ɽȪ����Ȫˮ���м��ʱ������1.0 L�ÿ�Ȫˮ�к���45.6mg Mg2+�����ʵ���Ũ��Ϊ
��2����KCL��CaCl2����ɵ�ij������У�K+��Ca2+�����ʵ���֮��Ϊ2��1����KCl��CaCl2�����ʵ���֮��Ϊ
1.90��10-3mol/L
1.90��10-3mol/L
������ʾ��1g=1000mg����2����KCL��CaCl2����ɵ�ij������У�K+��Ca2+�����ʵ���֮��Ϊ2��1����KCl��CaCl2�����ʵ���֮��Ϊ
2��1
2��1
���û�����е�CaCl2����������Ϊ42.7%
42.7%
����������1������n=
=cV���㣻
��2������n��KCl����n��CaCl2��=n��K+����n��Ca2+���������ʵ���֮�ȣ�����m=nM����������ϵ��������������������
| m |
| M |
��2������n��KCl����n��CaCl2��=n��K+����n��Ca2+���������ʵ���֮�ȣ�����m=nM����������ϵ��������������������
����⣺��1��n��Mg2+��=
=1.9��10-3mol��c��Mg2+��=
=1.90��10-3mol/L��
�ʴ�Ϊ��1.90��10-3mol/L��
��2����KCl��CaCl2�Ļ�ѧʽ��֪��n��KCl����n��CaCl2��=n��K+����n��Ca2+��=2��1��
��KClΪ2mol����CaCl2Ϊ1mol��
��m��KCl��=2mol��74.5g/mol=149g��m��CaCl2��=1mol��111g/mol=111g��
���ԣ��û�����е�CaCl2����������Ϊ
��100%=42.7%��
�ʴ�Ϊ��2��1��42.7%��
| 45.6��10-3g |
| 24g/mol |
| 1.9��10-3mol |
| 1.0L |
�ʴ�Ϊ��1.90��10-3mol/L��
��2����KCl��CaCl2�Ļ�ѧʽ��֪��n��KCl����n��CaCl2��=n��K+����n��Ca2+��=2��1��
��KClΪ2mol����CaCl2Ϊ1mol��
��m��KCl��=2mol��74.5g/mol=149g��m��CaCl2��=1mol��111g/mol=111g��
���ԣ��û�����е�CaCl2����������Ϊ
| 111g |
| 111g+149g |
�ʴ�Ϊ��2��1��42.7%��
���������⿼�����ʵ�������ؼ��㣬��Ŀ�ѶȲ���ע������������ļ��㹫ʽ�����ã�

��ϰ��ϵ�д�
 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ