��Ŀ����
8��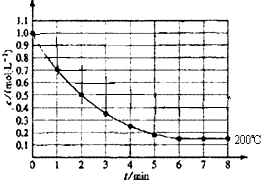 ��һ�ܱ������з���1molX��g��������Ӧ��X��g��?4Y��g��+Z��g������ͼ��ʾ�� 200��ʱ��X��Ũ����ʱ��仯�����ߣ�
��һ�ܱ������з���1molX��g��������Ӧ��X��g��?4Y��g��+Z��g������ͼ��ʾ�� 200��ʱ��X��Ũ����ʱ��仯�����ߣ���1��5min����Y��ʾ��ƽ����Ӧ����Ϊ0.64mol/��L��min������
��2���÷�Ӧ��6minʱ�̴ﵽƽ�⣮
��3����7minʱ�̣�V������=V���棩�����������������=������
��4���ڵ�8minʱ��Z��Ũ��Ϊ0.85mol•L-1��
��5������˵����˵���÷�Ӧ�Ѵ�ƽ��״̬����a��c�����ţ���
a����λʱ������0.1molX��ͬʱ������0.4molY
b����ͬʱ��������0.1molZ��ͬʱ������0.4molY
c����Ӧ��ϵ��X��Y��Z��Ũ�ȶ����ֲ���
d����Ӧ��ϵ�е��κ����ʶ����ٷ�����Ӧ��
���� ��1������v=$\frac{��c}{��t}$���м�����⣻
��2����ͼ��֪����6minʱX �����ʵ���Ũ�ȱ��ֲ��䣬��Ӧ��ƽ��״̬��
��3����7minʱ�̣���ƽ��״̬��
��4����8minʱ��Y��Ũ��Ϊ0.15mol/L����μӷ�Ӧ��Y��Ũ��=��1.0-0.15��mol/L=0.85mol/L��ͬһ��Ӧ��ͬһʱ����ڲμӷ�Ӧ�ĸ����ʵ�Ũ�ȱ仯��֮�ȵ����������֮�ȣ��ݴ˼���ZŨ�ȣ�
��5����ƽ��״̬ʱ���淴Ӧ������ȣ�����ֵ�Ũ�ȱ��ֲ��䣬�ɴ˷������
��� �⣺��1��v��X��=$\frac{��c}{��t}$=$\frac{1-0.2}{5}$=0.16mol/��L��min������v��Y��=4v��X��=4��0.16=0.64mol/��L��min�����ʴ�Ϊ��0.64mol/��L��min����
��2����ͼ��֪����6minʱX �����ʵ���Ũ�ȱ��ֲ��䣬��Ӧ��ƽ��״̬���ʴ�Ϊ��6min��
��3����7minʱ�̣���ƽ��״̬��V������=V���棩���ʴ�Ϊ��=��
��4����8minʱ��Y��Ũ��Ϊ0.15mol/L����μӷ�Ӧ��Y��Ũ��=��1.0-0.15��mol/L=0.85mol/L��ͬһ��Ӧ��ͬһʱ����ڲμӷ�Ӧ�ĸ����ʵ�Ũ�ȱ仯��֮�ȵ����������֮�ȣ�����ZŨ�ȱ仯��=XŨ�ȱ仯��=0.85mol/L��Z���������ƽ��ʱZŨ��Ϊ0.85 mol•L-1���ʴ�Ϊ��0.85 mol•L-1��
��5��a����λʱ������0.1molX�ĵ�Ч������0.4molY��ͬʱ����0.4molY�����淴Ӧ������ȣ���ƽ��״̬������ȷ��
b��ֻҪ��Ӧ��������ͬʱ��������0.1molZ��ͬʱ����0.4molY���ɣ����Բ�һ��ƽ�⣬�ʴ���
c����Ӧ��ϵ��X��Y��Z��Ũ�ȶ����ֲ��䣬˵�������ʵ������䷴Ӧ��ƽ��״̬������ȷ��
d����ѧ��Ӧ�Ƕ�̬ƽ�⣬ƽ��ʱ���ڽ��У��ʴ���ѡa��c��
���� ���⿼�黯ѧƽ���йؼ��㡢��ѧƽ��״̬�жϵ�֪ʶ�㣬Ϊ��Ƶ���㣬��ȷ��Ӧ����ʽ�и�����������ϵ�ǽⱾ��ؼ�����Ŀ�ѶȲ���

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�| A�� | H2O | B�� | CH3OH | C�� | CS2 | D�� | CH3COOH |
| A�� | 18 g H2O�����ķ�����ΪNA | |
| B�� | 22.4 L H2O�����ķ�����ΪNA | |
| C�� | NA����ԭ�ӵ�����Ϊ2 g | |
| D�� | 1 mol NaCl�к�0.5NA��Na+��0.5NA��Cl- |
 ij��ɫ��ҺM���ܺ�������OH-��HCO3-��CO32-��SO42-��SiO32-��AlO2-��NH4+��MnO4-��Cu2+��Mg2+��Na+��Fe3+�е������֣�ȡһ������M��Һ�μ����ᣬ�������������ʵ�������������Ĺ�ϵ��ͼ��ʾ��HCO3-��Al3+��AlO2-��Ҫ��Ӧ�������棩�������ж���ȷ���ǣ�������
ij��ɫ��ҺM���ܺ�������OH-��HCO3-��CO32-��SO42-��SiO32-��AlO2-��NH4+��MnO4-��Cu2+��Mg2+��Na+��Fe3+�е������֣�ȡһ������M��Һ�μ����ᣬ�������������ʵ�������������Ĺ�ϵ��ͼ��ʾ��HCO3-��Al3+��AlO2-��Ҫ��Ӧ�������棩�������ж���ȷ���ǣ�������| A�� | ԭ��Һ�п��ܺ���NH4+��SO42- | |
| B�� | ������Һ�����ٺ�2������ | |
| C�� | ԭ��Һ��n��NaAlO2����n��Na2CO3��=1��1 | |
| D�� | �μ������ʼ�η�����Ӧ�����ӷ���ʽ�ǣ�CO32-+H+�THCO3- |
| A�� | ���ˮ����μ�����������FeCl3��Һ�����Ƶ�Fe��OH��3���� | |
| B�� | ������ˮ������Al��OH��3���壬��������ˮ�� | |
| C�� | ��ˮ�еμ���������FeCl3��Һ���γɴ���Ľ��壬����������ǿ | |
| D�� | ��ѪҺ���������˽�������� |
 ��
�� ��
��