��Ŀ����
3����ϩ����Ҫ�Ļ���ԭ�ϣ�һ�������¿ɷ�������ת����
��֪��
R-X$\stackrel{NaCN}{��}$R-CN$\stackrel{H_{2}O/H+}{��}$RCOOH
R-CH2COOH$��_{P}^{Br_{2}}$

�ش��������⣺
��1�����»������к����Ȼ�����ABC��
A��������B B��������C C��������D D��������E
��2��B��ͬ���칹���ж��֣�д�����м��ܷ���������Ӧ�����ܷ���������Ӧ��2��ͬ���칹��Ľṹ��ʽ��HOCH2CH2CH2CHO����CH3CH��OH��CH2CHO��CH3CH2CH��OH��CHO����CH3��2C��OH��CHO��HOCH2CH��CH3��CHO��д��������2������
��3��д����̼����������D��Ϊͬϵ����л���Ľṹ��ʽHOOC-COOH��д��D�������Ҵ�������Ӧ�Ļ�ѧ����ʽ
 ��
��
���� ��ϩ��HBr�����ӳɷ�Ӧ����A��AΪ����飬A��KCN����ȡ����Ӧ������ ����AΪ
����AΪ ������ϢR-CNˮ��õ�RCOOH����֪BΪ
������ϢR-CNˮ��õ�RCOOH����֪BΪ ��B����ȡ����Ӧ����
��B����ȡ����Ӧ���� ��
�� ��KCN����ȡ����Ӧ����CΪ
��KCN����ȡ����Ӧ����CΪ ��Cˮ������DΪ
��Cˮ������DΪ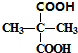 ��
��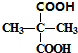 ���������Ҵ�����������Ӧ����E����EΪ
���������Ҵ�����������Ӧ����E����EΪ ���ݴ˽��
���ݴ˽��
��� �⣺��ϩ��HBr�����ӳɷ�Ӧ����A��AΪ����飬A��KCN����ȡ����Ӧ������ ����AΪ
����AΪ ������ϢR-CNˮ��õ�RCOOH����֪BΪ
������ϢR-CNˮ��õ�RCOOH����֪BΪ ��B����ȡ����Ӧ����
��B����ȡ����Ӧ���� ��
�� ��KCN����ȡ����Ӧ����CΪ
��KCN����ȡ����Ӧ����CΪ ��Cˮ������DΪ
��Cˮ������DΪ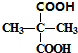 ��
��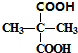 ���������Ҵ�����������Ӧ����E����EΪ
���������Ҵ�����������Ӧ����E����EΪ ��
��
��1��AΪ ������ϢR-CNˮ��õ�RCOOH����֪BΪ
������ϢR-CNˮ��õ�RCOOH����֪BΪ ��B����ȡ����Ӧ����
��B����ȡ����Ӧ���� ��
�� ��KCN����ȡ����Ӧ����CΪ
��KCN����ȡ����Ӧ����CΪ ��Cˮ������DΪ
��Cˮ������DΪ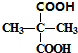 ��
��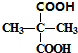 ���������Ҵ�����������Ӧ����E����EΪ
���������Ҵ�����������Ӧ����E����EΪ ����֪�����Ȼ�����BCD��
����֪�����Ȼ�����BCD��
�ʴ�Ϊ��ABC��
��2�� ��ͬ���칹���ж��֣����м��ܷ���������Ӧ�����ܷ���������Ӧ��˵�������к���ȩ��-CHO���ǻ�-OH�����Ͻṹ���л���Ϊ��HOCH2CH2CH2CHO��CH3CH��OH��CH2CHO��CH3CH2CH��OH��CHO����CH3��2C��OH��CHO��HOCH2CH��CH3��CHO��
��ͬ���칹���ж��֣����м��ܷ���������Ӧ�����ܷ���������Ӧ��˵�������к���ȩ��-CHO���ǻ�-OH�����Ͻṹ���л���Ϊ��HOCH2CH2CH2CHO��CH3CH��OH��CH2CHO��CH3CH2CH��OH��CHO����CH3��2C��OH��CHO��HOCH2CH��CH3��CHO��
�ʴ�Ϊ��HOCH2CH2CH2CHO��CH3CH��OH��CH2CHO��CH3CH2CH��OH��CHO����CH3��2C��OH��CHO��HOCH2CH��CH3��CHO ��д��������2������
��3��DΪ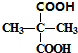 ���京C���ٵ�ͬϵ��Ϊ�Ҷ��ᣬ�ṹ��ʽΪHOOC-COOH��
���京C���ٵ�ͬϵ��Ϊ�Ҷ��ᣬ�ṹ��ʽΪHOOC-COOH��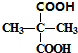 ���������Ҵ�����������Ӧ����E����EΪ
���������Ҵ�����������Ӧ����E����EΪ ����ѧ����ʽΪ
����ѧ����ʽΪ ��
��
�ʴ�Ϊ��HOOC-COOH�� ��
��
���� ���⿼���л��ƶ���ϳɣ��漰ϩ����±��������������������Լ�������Ϣ���еķ�Ӧ�ȣ��Ƕ��л�������֪ʶ���ۺϿ��飬ע�������л���ṹ����Ӧ���������ƶϣ��ܽϺõĿ��鿼������ѧ������˼ά�������Ǹ߿��ȵ����ͣ��Ѷ��еȣ�

 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�| A�� | ˮ�������c��OH�����ۣ��٣��� | |
| B�� | ��Һ��pH���ڣ��ۣ��� | |
| C�� | �ٺ͢ڵ������Ϻ����Һ��c��Na+���Tc��H2C03��+c��HC03��+c��C032-�� | |
| D�� | �ٺ͢۵������Ϻ����Һ��c��Na+����c��HC03-����c��C032-����c��OH-����c��H+�� |
| A�� | CH3CH2OH?CH2=CH2��g��+H2O��g������H��0 | |
| B�� | CO2��g��+H2��g��?CO��g��+H2O��g������H��0 | |
| C�� | CO2��g��+2NH3�� g��?CO��NH2��2��s��+H2O��g������H��0 | |
| D�� | 2C6H5CH2CH3��g��+O2��g��?2C6H-5CH=CH2��g��+2H2O��g������H��0 |
TiO2��s��+2Cl2��g���TTiCl4��l��+O2��g����H=+140.5kJ?mol-1
C��s��ʯī��+O2��g���TCO2��g����H=-110.5kJ?mol-1
��ӦTiO2��s��+2Cl2��g��+C��s��ʯī���TTiCl4��l��+CO2��g���ġ�H�ǣ�������
| A�� | +30.0kJ?mol-1 | B�� | -80.5kJ?mol-1 | C�� | -30.0kJ?mol-1 | D�� | +80.5kJ?mol-1 |
| A�� | C2H6��C3H8 | B�� | CH3-CH2-CH2-CH3�� | ||
| C�� | 1H��2H | D�� | O2��O3 |
| A�� | ����FeSO4��Һʱ��������������ۺ�ϡ���ᣬ�ȷ�ֹ����������ˮ�� | |
| B�� | ��ˮ�м���������¶���ʹ����ƽ�������ƶ�����ˮ�����ӻ����� | |
| C�� | �ڵ��з�̪��Na2CO3��Һ�У�����BaCl2��Һ���ɫ��ȥ����֤Na2CO3��Һ�д���ˮ��ƽ�� | |
| D�� | ��֪I3-?I2+I-����ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ� |

 �ҹ��Ŵ���ͭ�����վ�տ���кܸߵ�������ֵ����ʷ��ֵ������������ͭ������ܵ�������ʴ���ʶ���������ͷ���������Ҫ���壮
�ҹ��Ŵ���ͭ�����վ�տ���кܸߵ�������ֵ����ʷ��ֵ������������ͭ������ܵ�������ʴ���ʶ���������ͷ���������Ҫ���壮