��Ŀ����
17��ij��A 0.2mol�������г��ȼ�պ����ɻ�����B��C��1.2mol���Իش���1����A�ķ���ʽ��C6H12��
��2����ȡһ��������A���ȼ�պ�����B��C��3mol������42g��A�μ��˷�Ӧ��ȼ��ʱ���ı�״���µ�����100.8L��
��3������A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ�ȴ���ֻ��һ�֣�����A�Ľṹ��ʽ��
 ��
����4������A��ʹ��ˮ��ɫ���ڴ�����������H2�����ӳɷ�Ӧ������
 ����A�Ľṹ��ʽΪ��CH3��3CCH=CH2��
����A�Ľṹ��ʽΪ��CH3��3CCH=CH2����5������A��ʹ��ˮ��ɫ���ҷ���������̼ԭ�ӹ�ƽ�棬��A�Ľṹ��ʽΪ��CH3��2C=C��CH3��2��
��6����A��2��̼ԭ�ӵ�A��ϩ����ͬϵ���ͬ���칹�干��3�֣�
���� ��1��������C��H����Ԫ�أ�ij��A0.2mol�������ij��ȼ�պ����ɻ�����B��C��1.2mol��������CO2��H2O��1.2mol����1mol���к���6molC��12molHԭ�ӣ�A����ʽΪC6H12��
��2������ȼ�շ���ʽ����A�����ʵ������������������ʵ������ٸ���m=nM������A������������V=nVm�������������������
��3������A����ʹ��ˮ��ɫ��˵���л����в���C=C�����ţ�����һ�������£�������������ȡ����Ӧ����һ�ȴ���ֻ��һ�֣�ӦΪ�����飻
��4������A��ʹ��ˮ��ɫ���ڴ�����������H2�ӳ����� ��˵�������к���1��C=C������������̼ԭ�Ӷ�����Hԭ��ΪC=C˫��λ�ã��ݴ�ȷ��A�Ľṹ��ʽ��
��˵�������к���1��C=C������������̼ԭ�Ӷ�����Hԭ��ΪC=C˫��λ�ã��ݴ�ȷ��A�Ľṹ��ʽ��
��5������A��ʹ��ˮ��ɫ���ҷ���������̼ԭ�ӹ�ƽ�棬��C=C˫���в�����Cԭ�������ĸ������ݴ�ȷ��A�Ľṹ��ʽ��
��6����A������̼ԭ�ӵ�A��ϩ��ͬϵ�����ʽΪC4H8��
��� �⣺��1��������C��H����Ԫ�أ�ij��A0.2mol�������ij��ȼ�պ����ɻ�����B��C��1.2mol��������CO2��H2O��1.2mol����1mol���к���6molC��12molHԭ�ӣ�����ʽΪC6H12��
�ʴ�Ϊ��C6H12��
��2��C6H12��ȫȼ�գ�����3molCO2��H2O����
C6H12+9O2$\frac{\underline{\;��ȼ\;}}{\;}$6CO2+6H2O��
1mol 9mol 6mol 6mol
0.5mol 4.5mol 3mol 3mol
������3molCO2��H2Oʱ����Ҫ0.5molC6H12��m��C6H12��=0.5mol��84g/mol=42g��
��Ҫ���������ΪV��O2��=4.5mol��22.4L/mol=100.8L��
�ʴ�Ϊ��42��100.8��
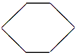
��3������A����ʹ��ˮ��ɫ��˵���л����в���C=C�����ţ�����һ�������£�������������ȡ����Ӧ����һ�ȴ���ֻ��һ�֣�ӦΪ�����飬�ṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4������A��ʹ��ˮ��ɫ���ڴ�����������H2�ӳ����� ��˵�������к���1��C=C������������̼ԭ�Ӷ�����Hԭ��ΪC=C˫��λ�ã���A�Ľṹ��ʽΪ����CH3��3CCH=CH2��
��˵�������к���1��C=C������������̼ԭ�Ӷ�����Hԭ��ΪC=C˫��λ�ã���A�Ľṹ��ʽΪ����CH3��3CCH=CH2��
�ʴ�Ϊ����CH3��3CCH=CH2��
��5������A��ʹ��ˮ��ɫ���ҷ���������̼ԭ�ӹ�ƽ�棬��C=C˫���в�����Cԭ�������ĸ�������A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2��
�ʴ�Ϊ����CH3��2C=C��CH3��2��
��6����A������̼ԭ�ӵ�A��ϩ��ͬϵ�����ʽΪC4H8����ϩ��ͬ���칹���У�CH2=CHCH2CH3��CH3CH=CHCH3��CH2=C��CH3��2������3�ֶ�ϩ��ͬ���칹�壬
�ʴ�Ϊ��3��
���� ���⿼���л�����ƶϣ��漰����ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ�Ϊ��Ƶ���㣬���չ����������ʵĹ�ϵΪ���Ĺؼ��������л���Ľṹ�ķ������ƶ������Ŀ��飮

 ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�| A�� | ${\;}_{6}^{14}$C��${\;}_{6}^{12}$C��ͬһ�ֺ��� | |
| B�� | ���Ͱ���Ϊͬ�������� | |
| C�� | CH3COOCH2CH3��CH3CH2COOCH3�Dz�ͬ���� | |
| D�� | CH3CH2OH�ɿ�������-C2H5��-OH��� |
| A�� | ����Լռ����������71%�����Ե����ϲ�ȱˮ | |
| B�� | ��ˮ�������ijɱ���� | |
| C�� | ��ˮ��������Ҫ�����������������������ӽ������� | |
| D�� | ����˵������ȷ |
| A�� | ������ĵ��뷽��ʽΪ��HClO�TH++ClO- | |
| B�� | c��H+������1��10-7mol•L-1����Һһ����������Һ | |
| C�� | ��CH3COONa��Һ�У�c��CH3COO-����c��Na+�� | |
| D�� | 0.2mol•L-1CH3COOH��Һ�е�c��H+����0.1mol•L-1 HCl��Һ�е�c��H+����2�� |
| A�� | ʳ�Ρ���ˮ | B�� | �ȡ��塢�� | C�� | �ơ�þ���� | D�� | �ռ���� |

 ��
�� +2H2O��
+2H2O�� ��
��