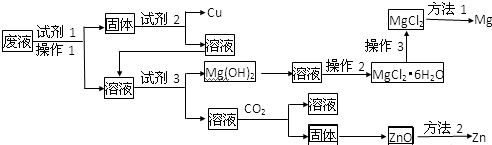
��Ŀ����
11��Bͬѧ����ʵ�����÷���м��������ͭ����ȡ���;�ˮ��Na2FeO4��������ͼ��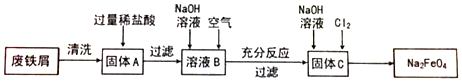
��ش��������⣮
��1�������A�м���ϡ���������Ӧ�����ӷ���ʽ��Fe+2H+=Fe2++H2������Ӧ��õ�����Һ����ϳ�ʱ�䱣�棬Ӧ��ȡ�Ĵ�ʩ������Һ�м����������ۣ�
��2����ҺB�е���������Fe2+��H+������ҺB�м���NaOH��Һ��֮��ͨ����������裬�۲쵽���������Ȳ�����ɫ��������ɫ����Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ��ͨ�����������ѧ��Ӧ�Ļ�ѧ����ʽ��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��
��3�������C�м���NaOH��Һ��ͨ��Cl2����ȡNa2FeO4���йط�Ӧ�Ļ�ѧ����ʽ��2Fe��OH��3+10NaOH+3Cl2=2Na2FeO4+6NaCl+8H2O��
��4��Na2FeO4��Fe�Ļ��ϼ���+6����������ԭ�Ƕȷ�����Na2FeO4���������ԣ�����Na2FeO4��ˮʱ�����˿ɳ�ȥˮ���������ʣ�������ɱ��������
���� �÷���м��������ͭ����ȡ���;�ˮ��Na2FeO4�����̣���ϴ����м����������ۣ����������ϡ���ᣬ������ϡ���ᣬͭ���ܣ����˵õ��Ȼ�������HCl�Ļ����ҺB�������м�������������Һ���к����ᣬ������������������ͨ�������������Ϊ�������������˵õ��Ĺ���CΪ���������������������ƺ������õ���ƷNa2FeO4���ݴ˷�����
��� �⣺��1������A�м���ϡ���������Ӧ�����ӷ���ʽ�ǣ�Fe+2H+=Fe2++H2�������������ױ����������賤ʱ�䱣�棬�ɼ��뻹ԭ�����ۣ�
�ʴ�Ϊ��Fe+2H+=Fe2++H2��������Һ�м����������ۣ�
��2����ҺBΪ�Ȼ������Ͷ�������ᣬ������Ϊ��Fe2+��H+����B��Һ�м����������ƣ������ɰ�ɫ����������������������������������������Ӧ4Fe��OH��2+O2+2H2O�T4Fe��OH��3�����ɺ��ɫ����������������������Ϊ���Ȳ�����ɫ��������ɫ����Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��
�ʴ�Ϊ��Fe2+��H+���Ȳ�����ɫ��������ɫ����Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��
��3�������������м���NaOH��Һ��ͨ��Cl2����ȡNa2FeO4����ӦΪ��2 Fe��OH��3+10NaOH+3Cl2=2Na2FeO4+6NaCl+8H2O��
�ʴ�Ϊ��2 Fe��OH��3+10NaOH+3Cl2=2Na2FeO4+6NaCl+8H2O��
��4��Na2FeO4����Ԫ��+1�ۣ���Ԫ��-2�ۣ����ϼ۴�����Ϊ0����Fe�Ļ��ϼ���+6�ۣ���Ԫ��+6�ۣ��õ��ӻ��ϼ۽��ͣ����������ԣ���ɱ��������
�ʴ�Ϊ��+6��������ɱ��������
���� ���⿼�������ʵ��Ʊ���Χ����չ�����漰������ԭ��Ӧ����ʽ����д�����̵ķ����ȣ��������������ǹؼ�����Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� |  ���������ζ | B�� |  ��Ũ�������CO2 | ||
| C�� |  ������ƿ��ת��Һ�� | D�� | 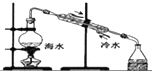 �ú�ˮ��ȡ��ˮ |
�ٻ���̿������ɫ���� ����ˮƯ����ɫ����
�۹�������¶���ڿ����� �ܽ�����ͨ��ˮ�У���Һ��dz����ɫ
�ݹ����������Ƽ��뺬��̪����Һ����Һ�ȱ�����ɫ ��������ɫ��Ӧ����NaCl��KCl��
| A�� | �٢ڢۢ� | B�� | �٢ڢܢ� | C�� | �ڢۢܢ� | D�� | �ۢܢݢ� |
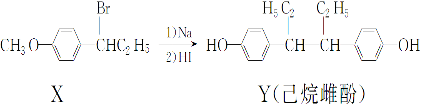
����������ȷ���ǣ�������
| A�� | ��NaOH ˮ��Һ�м��ȣ�������X �ɷ�����ȥ��Ӧ | |
| B�� | ��һ�������£�������Y����Ũ��ˮ����ȡ����Ӧ | |
| C�� | ��FeCl3��Һ���ܼ�����X��Y | |
| D�� | ������Y�в���������̼ԭ�� |
| A�� | c��SO42-��=c��HS-��=c��K+����c��OH-��=c��H+�� | |
| B�� | c��Na+����c��K+����c��S2-����c��H+����c��OH-�� | |
| C�� | c��Na+��=c��S2-��+c��HS-��+c��H2S��+c��SO42-�� | |
| D�� | c��K+��+c��Na+��+c��H+��=c��SO42-��+c��S2-��+c��HS-��+c��OH-�� |
| A�� | A��BԪ���γɵ�һϵ�л������У�����AԪ���������������ֵΪ25% | |
| B�� | ����Ԫ���е縺��������B | |
| C�� | C���γɵ���̬�⻯�����ͬ����Ԫ�ص���̬�⻯���зе���� | |
| D�� | ����Ԫ���е�һ��������С����D |
| A�� | ȷ��ȡ20.00mL���������Һ����ѡ��25mL��ʽ�ζ��� | |
| B�� | ��ˮ���ȣ�Kw����pH���� | |
| C�� | �ö��Ե缫���1LŨ�Ⱦ�Ϊ2mol/L��AgNO3��Cu��NO3��2�Ļ����Һ������0.2 mol ����ת��ʱ����������6.4g���� | |
| D�� | NaAlO2��ˮ��Һ������Ũ�����������պ��ܵõ�NaAlO2���� |
��NaHCO3 ��Al2O3 ��Al��OH��3 ��Al ��AlCl3 ��NaAlO2��
| A�� | �ۢܢ� | B�� | �ڢۢ� | C�� | �٢ۢܢ� | D�� | �٢ڢۢ� |