��Ŀ����
17�� ������أ�K2FeO4�����м�ǿ�������ԣ�����Ϊˮ��������������ز��ϣ�
������أ�K2FeO4�����м�ǿ�������ԣ�����Ϊˮ��������������ز��ϣ���1��FeO42-��ˮ��Ӧ�ķ���ʽΪ4FeO42-+10H2O?4Fe��OH��3+8OH-+3O2����K2FeO4�ڴ���ˮ�Ĺ�����������������ܹ�����ɱ����ͬʱFeO42-����ԭ��Fe3+��Fe3+ˮ���γ�Fe��OH��3���壬�ܹ�����ˮ���������ʣ�
��2����MnO2-Zn������ƣ�K2FeO4-ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ������Ϊ�������ϣ���缫��ӦʽΪFeO42-+3e��+4H2O=Fe��OH��3+5OH-��
��3��������K2FeO4���Ƴ�c��FeO42-��=1.0��10-3mol•L-1��1.0mmol•L-1����������FeO42-��ˮ��Һ�еĴ�����̬��ͼ��ʾ������˵����ȷ����BD������ĸ��
A��������Һ�������α仯����Ԫ�ض���4�ִ�����̬
B���ı���Һ��pH������Һ��pH=10����pH=4�Ĺ����У�HFeO4-�ķֲ�������������С
C����pH=8��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��H2FeO4+OH-=HFeO4-+H2O
D��pHԼΪ2.5 ʱ����Һ��H3FeO4+��HFeO4-�����൱
��4��HFeO4-�TH++FeO42-�ĵ���ƽ�ⳣ������ʽΪK������ֵ�ӽ�C������ĸ����
A��10-2.5 B��10-6 C��10-7 D��10-10
��5��25��ʱ��CaFeO4��Ksp=4.536��10-9����Ҫʹ100mL��1.0��10-3mol•L-1��K2FeO4��Һ�е�c��FeO42- ����ȫ������һ����Ϊ����Ũ��С��1��10-5mol•L-1ʱ��Ϊ��ȫ���������������������Һ��Ca2+Ũ������Ϊ4.536��10-5mol•L-1��
���� ��1��������أ�K2FeO4�����м�ǿ�������ԣ���һ��������ˮ����������ɱ���������ã��γɽ�����������������ʵ����ã�
��2��������FeO42-������ԭ��Ӧ���������������缫��ӦʽΪ��FeO42-+3e��+4H2O=Fe��OH��3+5OH-��
��3��A����ͬPHֵʱ����Һ����Ԫ�صĴ�����̬����������ͬ��������PHֵ����6ʱ����ֻ��������̬��
B������ͼƬ֪���ı���Һ��pH������Һ��pH=10����pH=4�Ĺ����У�HFeO4-�ķֲ�������������С��
C����pH=8��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��HFeO4-+OH-=FeO42-+H2O
D������ͼƬ֪pHԼΪ2.5 ʱ����Һ��H3FeO4+��HFeO4-�����൱��
��4��ƽ�ⳣ������������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ�
��5�������ܶȻ��������㣮
��� �⣺��1��������أ�K2FeO4�����м�ǿ�������ԣ���һ��������ˮ����������ɱ���������ã�ͬʱFeO42- ����ԭ��Fe3+��Fe3+ˮ���γ�Fe��OH��3���壬���������������ʵ����ã�
�ʴ�Ϊ���ܹ�����ɱ����ͬʱFeO42- ����ԭ��Fe3+��Fe3+ˮ���γ�Fe��OH��3���壬�ܹ�����ˮ���������ʣ�
��2��������FeO42-������ԭ��Ӧ���������������缫��ӦʽΪ��FeO42-+3e��+4H2O=Fe��OH��3+5OH-���ʴ�Ϊ��FeO42-+3e��+4H2O=Fe��OH��3+5OH-��
��3��A����ͬPHֵʱ����Һ����Ԫ�صĴ�����̬����������ͬ��������PHֵ����6ʱ����ֻ��������̬����A����
B������ͼƬ֪���ı���Һ��pH������Һ��pH=10����pH=4�Ĺ����У�HFeO4-�ķֲ�������������С������ȷ��
C����pH=8��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��HFeO4-+OH-=FeO42-+H2O���ʴ���
D������ͼƬ֪pHԼΪ2.5 ʱ����Һ��H3FeO4+��HFeO4-�����൱������ȷ��
��ѡBD��
��4��HFeO4-?H++FeO42-�ĵ���ƽ�ⳣ������ʽΪK=$\frac{c��{H}^{+}��•c��Fe{O}_{4}^{2-}��}{c��HFe{O}_{4}^{-}��}$������ͼ��֪����HFeO4-��FeO42-
Ũ�����ʱ����Һ��������Ũ�Ƚӽ����ԣ�����K�ӽ�10-7��
�ʴ�Ϊ��C��
��5��25��ʱ��CaFeO4��Ksp=4.536��10-9����Ҫʹ100mL1.0��10-3mol•L-1��K2FeO4��Һ�е�c��FeO42- ����ȫ����������������Ҫ�����Ca��OH��2�����ʵ���=$\frac{{K}_{sp}}{c��Fe{O}_{4}^{2-}��}$=$\frac{4.536��1{0}^{-9}}{1.0��1{0}^{-5}}$=4.536��10-5���ʴ�Ϊ��4.536��10-5mol•L-1��
���� ���⿼�����ܶȻ�������������أ�K2FeO4�������ʵ�֪ʶ�㣬�Ѷ��еȣ��ѵ����ܶȻ��������йؼ��㣬����Һ������Ũ��Ϊ1��10-5mol/Lʱ��Ϊ������ȫ������Ϊ�״��㣮

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��Ӧ������������������������ʱ���÷�Ӧһ�����ܷ��� | |
| B�� | ��ѧ���ļ���Խ�����ʵ�����Խ�� | |
| C�� | һ����Ӧ���ʱ���Ӧ��������ͷ�Ӧ�����ĸı�������ı� | |
| D�� | Ӧ�ø�˹���ɣ��ɼ���ijЩ����ֱ�Ӳ����ķ�Ӧ�ʱ� |
| A�� |  | B�� |  | ||
| C�� |  | D�� |  |
| A�� | 3�� | B�� | 4�� | C�� | 6�� | D�� | 8�� |
| A�� | � | B�� | �� | C�� | �� | D�� | � |

| A�� | �ڶ����ڵĺ˵����������ϼ�֮��Ĺ�ϵ | |
| B�� | ������̼ԭ������X������ԭ������Y���Ĺ�ϵ | |
| C�� | �¶ȴ���100��ʱ��CH4��C2H4�Ļ�������������г��ȼ�գ�ͬ��ͬѹ��ȼ��ǰ����뷴Ӧ����������֮�ͣ�X������������������֮�ͣ�Y���Ĺ�ϵ | |
| D�� | ȼ��һ������C2H4��C3H6�Ļ�����壬����O2�����ʵ�����Y����C3H6������������X���Ĺ�ϵ |
| A�� | c��Ba2+����c��OH-����c��Na+����c��CO32-�� | B�� | c��OH-����c��Na+����c��Ba2+����c��CO32-�� | ||
| C�� | c��OH-����c��Ba2+����c��Na+����c��CO32-�� | D�� | c��Na+����c��OH-����c��Ba2+����c��CO32-�� |
 ijͬѧ�����ͼװ�ã��о��ǽ���Ԫ�����ʱ仯���ɣ�
ijͬѧ�����ͼװ�ã��о��ǽ���Ԫ�����ʱ仯���ɣ�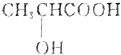 ����һ�������칹�壬��Ϊ������к���һ������̼ԭ�ӣ�
����һ�������칹�壬��Ϊ������к���һ������̼ԭ�ӣ�