��Ŀ����
5����ʯ��Ϊԭ������������Ʒ������˵����ȷ���ǣ�������ʯ��$\stackrel{��}{��}$�����Ʒ$\stackrel{��}{��}$��ϩ$\stackrel{��}{��}$CH2BrCH2Br��
| A�� | �٢ڹ����ж�ֻ�����������仯 | |
| B�� | ʯ����Ҫ���ɸ�������������������������ɵĻ���� | |
| C�� | ʯ�͵ķ����Ʒ�в��ܵõ����͡����� | |
| D�� | ���Ǽӳɷ�Ӧ������������1��2-������ |
���� A���ѻ����ѽ�ȹ����ǰѴ���ӵ���ת��ΪС��������
B��ʯ�͵���Ҫ�ɷ��Ǹ������������������������Ļ���
C����ʯ�ͼ��ȷ���ɵõ����͡�ú�͡����͡����͡�ʯ�����ܼ��͡�����Ȳ�Ʒ��
D����ϩ����ӳ�����1��2-�������飮
��� �⣺A���ѻ����ѽ�ȹ����ǰѴ���ӵ���ת��ΪС����������������ϩ���ɣ����Ԣڰ����ѻ����ѽ�ȹ��̣������������ɣ����ڻ�ѧ�仯����A����
B��ʯ�͵���Ҫ�ɷ��Ǹ������������������������Ļ�����ʯ����Ҫ��������ɵĻ�����B��ȷ��
C��ʯ���к��ж��ֳɷ֣���Щ�ɷֵķе㲻ͬ�����ݷе㲻ͬ�����Ǽ��ȷ���ɵõ����͡�ú�͡����͡����͡�ʯ�����ܼ��͡�����Ȳ�Ʒ����C����
D����ϩ����ӳ�����CH2BrCH2Br�����Ԣ��Ǽӳɷ�Ӧ��CH2BrCH2Br������Ϊ1��2-�������飬��D����
��ѡB��
���� ���⿼����ʯ�͵ijɷ֡�ʯ�͵��������̡��л���������ȣ����ڻ���֪ʶ�Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д�
�����Ŀ
19��������������ȷ���ǣ�������
| A�� | ֻ�зǽ���ԭ�Ӽ�����γɹ��ۼ� | |
| B�� | �л�ѧ�����ѵı仯���ڻ�ѧ�仯 | |
| C�� | �ɹ��ۼ��γɵ�����-���ǹ��ۻ�������� | |
| D�� | ���ӻ������п��ܺ��й��ۼ��������ۻ�������һ���������Ӽ� |
16����a mol C2H4��b mol H2���ܱ������У����ʵ������£���Ӧ�ﵽƽ��ʱ������p mol C2H6����������ƽ�������������壬����CO2��H2O���������������ʵ���Ӧ�ǣ�������
| A�� | ��3a+0.5b��mol | B�� | ��3a+0.5b��mol | C�� | ��3a+0.5b+3p��mol | D�� | ��3a+0.5b-3p��mol |
13����ͼ�Dz��ֶ�����Ԫ�ػ��ϼ���ԭ�������Ĺ�ϵͼ������˵����ȷ���ǣ�������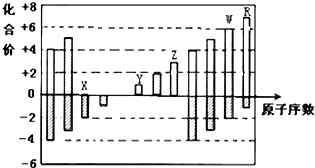

| A�� | ԭ�Ӱ뾶��Z��Y��X | |
| B�� | ��̬�⻯����ȶ��ԣ�R��W | |
| C�� | Y��Z��������������Ӧ��ˮ���������Ӧ | |
| D�� | WX3��ˮ��Ӧ�γɵĻ����������ӻ����� |
20���йػ�ѧ������ȷ���ǣ�������
| A�� | �����ʵ��ʽC2H402 | B�� | �۱�ϩ�Ľṹ��ʽ��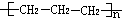 | ||
| C�� | �ǻ��ĵ���ʽ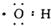 | D�� | ������ӵ����ģ�� |
10������˵����ȷ���ǣ�������
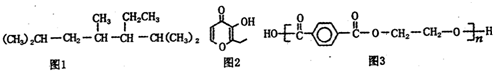

| A�� | ��ϵͳ������������ͼ1�������������2��4��6һ����-5-�һ����� | |
| B�� | ͼ2�л����һ�ַ�����ͬ���칹���ܷ���������Ӧ | |
| C�� | ͼ2�л�����ʹ���Ը��������Һ��ɫ | |
| D�� | ͼ3Ϊ�{���ӻ�����䵥��Ϊ�Ա���������Ҵ� |
17�����и����������仯�������ķ�Ӧ������ͬһ��Ӧ���͵��ǣ�������
���ɼױ��Ƽ������顢��������������
����ϩʹ��ˮ��ɫ����Ȳʹ���Ը��������Һ��ɫ
������ϩ�ƾ���ϩ���ɶ���ϩ�ƾ�-1��3-����ϩ
���ɱ������������ɱ����屽��
���ɼױ��Ƽ������顢��������������
����ϩʹ��ˮ��ɫ����Ȳʹ���Ը��������Һ��ɫ
������ϩ�ƾ���ϩ���ɶ���ϩ�ƾ�-1��3-����ϩ
���ɱ������������ɱ����屽��
| A�� | �ڢ� | B�� | �ۢ� | C�� | �٢� | D�� | �٢� |
14�����������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ���ǣ�������
| A�� | ��֪C��ʯī��s��=C�����ʯ��s����H��0������ʯ��ʯī�ȶ� | |
| B�� | ������ȼ����Ϊ285.5 kJ•mol-1����ˮ�ֽ���Ȼ�ѧ����ʽ2H2O��l��=2H2��g��+O2��g����H=+285.5 kJ•mol-1 | |
| C�� | ��֪ϡ��Һ�У�H+��aq��+OH-��aq��=H2O��l����H=-57.3 kJ•mol-1����Ũ������ϡNaOH��Һ��Ӧ����1 molˮʱ�ų�������Ϊ57.3 kJ | |
| D�� | �ܱ������У�9.6 g�����11.2 g���ۻ�ϼ�������������17.6 gʱ���ų�19.12 kJ��������Fe��s��+S��s��=FeS��s����H=-95.6 kJ•mol-1 |