��Ŀ����
ij����ѧϰС���ͬѧ�������ͼ��װ�ã�����֤SO2�������ԡ���ԭ�Ժ�Ư���ԣ�

�ش��������⣺
��1���������װ��C�������ԵIJ����ǣ��رջ���b��Ȼ�� �������� ��˵��װ��C���������ã�
��2����Na2SO3�����������Һ��ȡSO2���壬Ӧѡ�� ��ѡ��A����C���������巢��װ�ã���ѡ����һװ�õ������� ��
��3��С���ͬѧ��A��Cװ���е���һ����FeS�����ϡ������ȡH2S���壬��Ӧ�Ļ�ѧ����ʽΪ ��
��4��SO2����ͨ��Dװ��ʱ������ ��ͨ��Eװ��ʱ������ ��SO2��H2S��Bװ���з�Ӧ�������� ��
��5��F��ʢ�м�ʯ�ң��������� ��

�ش��������⣺
��1���������װ��C�������ԵIJ����ǣ��رջ���b��Ȼ��
��2����Na2SO3�����������Һ��ȡSO2���壬Ӧѡ��
��3��С���ͬѧ��A��Cװ���е���һ����FeS�����ϡ������ȡH2S���壬��Ӧ�Ļ�ѧ����ʽΪ
��4��SO2����ͨ��Dװ��ʱ������
��5��F��ʢ�м�ʯ�ң���������
���㣺̽������������ˮ��Ʒ����Һ�ķ�Ӧ
ר�⣺ʵ����
��������1�����װ�õ�������ԭ���Ǹ���װ�������ѹǿ���γ�ˮ�������ݣ��ݴ˽��
��2�����ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ã�
��3�����ݸ��ֽⷴӦ���ص㼴��д����ѧ����ʽ��
��4������SO2��ʹƷ����Һ�ĺ�ɫ��ȥ����������𣻸���SO2������������Һ��Ӧ����������𣻸���SO2+2H2S=3S+2H2O�����
��5��SO2�ж���Ⱦ�����������ŷŵ������У���ʯ��������SO2�����ã�
��2�����ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ã�
��3�����ݸ��ֽⷴӦ���ص㼴��д����ѧ����ʽ��
��4������SO2��ʹƷ����Һ�ĺ�ɫ��ȥ����������𣻸���SO2������������Һ��Ӧ����������𣻸���SO2+2H2S=3S+2H2O�����
��5��SO2�ж���Ⱦ�����������ŷŵ������У���ʯ��������SO2�����ã�
���
�⣺��1�����װ���������Ƿ����ã��رջ���b�����Թ��м�ˮ������ˮ�Ӳ���ȥ���Թ���һ�����ϵͳ�����������ã���֮�����Բ��ã�
�ʴ�Ϊ�����Թ��м�ˮ����ˮ�Ӳ���ȥ��
��2��ʵ�����ÿ�״��������������Ũ���ᷴӦ��ȡ�����������壬��Ӧ�Ļ�ѧ����ʽΪ��Na2SO3+H2SO4��Ũ��=Na2SO4+H2O+SO2����SO2�����ܶȱȿ������ж�������ˮ��Ӧ���������ᣬ��ȡ��������ķ�Ӧ��״̬�ͷ�Ӧ�������ڹ�Һ�����ͣ�����ѡ��ķ���װ����C�����շ����������ڹ�Һ���״̬�䲻����ȶ���ȡ�����壬�ҹ�������ǿ�״�ģ�ʵ�������������ƺ�������ȡ���������������Ƿ�ĩ״���壬���Բ��������շ�������ȡ��
�ʴ�Ϊ��C��Na2SO3������ˮ��SO2Ҳ������ˮ�������������շ��������ܵ�װ�ã�
��3����������FeS�������ϡ�����ڳ����·������ֽⷴӦ��FeS+H2SO4��ϡ���TFeSO4+H2S���������������������������
�ʴ�Ϊ��FeS+H2SO4��ϡ���TFeSO4+H2S����
��4��SO2��ʹƷ����Һ�ĺ�ɫ��ȥ������SO2����ͨ��Dװ��ʱ�����Ǻ�ɫ��ȥ�����������л�ԭ�ԣ����������ǿ�����ԣ����������������ܷ���5SO2+2MnO4+2H2O�T5SO42-+2Mn2++4H+��ɫ��ȥ��SO2��H2S�ܷ���SO2+2H2S=3S+2H2O������ƿ���е���ɫ��ĩ����ɫ��СҺ�Σ�
�ʴ�Ϊ����ɫ��ȥ����ɫ��ȥ�� ƿ���е���ɫ��ĩ����ɫ��СҺ�Σ�
��5��SO2�ж�������β������ֱ���ſգ�Ӧ��ѡ�ü����������գ����Լ�ʯ�ҵ�����������δ��Ӧ���SO2��
�ʴ�Ϊ������β����
�ʴ�Ϊ�����Թ��м�ˮ����ˮ�Ӳ���ȥ��
��2��ʵ�����ÿ�״��������������Ũ���ᷴӦ��ȡ�����������壬��Ӧ�Ļ�ѧ����ʽΪ��Na2SO3+H2SO4��Ũ��=Na2SO4+H2O+SO2����SO2�����ܶȱȿ������ж�������ˮ��Ӧ���������ᣬ��ȡ��������ķ�Ӧ��״̬�ͷ�Ӧ�������ڹ�Һ�����ͣ�����ѡ��ķ���װ����C�����շ����������ڹ�Һ���״̬�䲻����ȶ���ȡ�����壬�ҹ�������ǿ�״�ģ�ʵ�������������ƺ�������ȡ���������������Ƿ�ĩ״���壬���Բ��������շ�������ȡ��
�ʴ�Ϊ��C��Na2SO3������ˮ��SO2Ҳ������ˮ�������������շ��������ܵ�װ�ã�
��3����������FeS�������ϡ�����ڳ����·������ֽⷴӦ��FeS+H2SO4��ϡ���TFeSO4+H2S���������������������������
�ʴ�Ϊ��FeS+H2SO4��ϡ���TFeSO4+H2S����
��4��SO2��ʹƷ����Һ�ĺ�ɫ��ȥ������SO2����ͨ��Dװ��ʱ�����Ǻ�ɫ��ȥ�����������л�ԭ�ԣ����������ǿ�����ԣ����������������ܷ���5SO2+2MnO4+2H2O�T5SO42-+2Mn2++4H+��ɫ��ȥ��SO2��H2S�ܷ���SO2+2H2S=3S+2H2O������ƿ���е���ɫ��ĩ����ɫ��СҺ�Σ�
�ʴ�Ϊ����ɫ��ȥ����ɫ��ȥ�� ƿ���е���ɫ��ĩ����ɫ��СҺ�Σ�
��5��SO2�ж�������β������ֱ���ſգ�Ӧ��ѡ�ü����������գ����Լ�ʯ�ҵ�����������δ��Ӧ���SO2��
�ʴ�Ϊ������β����
���������⿼����SO2�����ʵ�ʵ����ƺ����ʷ����жϣ����ն��������������������ʡ�Ư���ԡ���ԭ�Ե��ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
ij��ɫ��Һ�м������۲��������������Һ�п϶��ܴ�������������ǣ�������
| A��Na+��NH4+��SO42-��NO3- |
| B��Na+��K+��Cl-��SO42- |
| C��Fe3+��Mg2+��S2-��SO42- |
| D��K+��Na+��AlO2-��MnO4- |
����������ԭ��Ӧ�е���ת�Ʒ������Ŀ����ȷ���ǣ�������
A�� |
B�� |
C�� |
D�� |
���ڼ�����������У�������ǣ�������
| A�������ֻ��һ�����ӣ���Ӧ����ʧȥ |
| B����˵���������۵����� |
| C����˵����������������ļ�����ǿ |
| D����˵�������Ӹ�ˮ��Ӧ���Ӿ��� |
 X��W��Y��Z��Ϊ����������Ԫ�أ�ԭ��������������Xԭ�ӵ�������������W��4����X��Y�����ڱ������λ����ͼ��
X��W��Y��Z��Ϊ����������Ԫ�أ�ԭ��������������Xԭ�ӵ�������������W��4����X��Y�����ڱ������λ����ͼ��
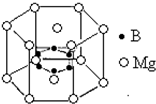 þ���ơ��ء��塢�������Ԫ����ÿ����ˮ�еĺ���������1mg�����ں�ˮ�еij���Ԫ�أ�
þ���ơ��ء��塢�������Ԫ����ÿ����ˮ�еĺ���������1mg�����ں�ˮ�еij���Ԫ�أ�