��Ŀ����
 þ���ơ��ء��塢�������Ԫ����ÿ����ˮ�еĺ���������1mg�����ں�ˮ�еij���Ԫ�أ�
þ���ơ��ء��塢�������Ԫ����ÿ����ˮ�еĺ���������1mg�����ں�ˮ�еij���Ԫ�أ���1��������ͬ���壬д����Ԫ��ԭ�ӵļ۵����Ų�ʽ
��2���ء��ơ��صĵ�һ�����ܴӴ�С��˳����
��3���ȽϷ����ƺ��廯�Ƶ��۵㣺������
��4���ü۲���ӶԻ��������ƶ�BF3�ռ乹��Ϊ
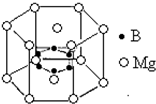
��5��2001������������þ������ˢ���˽��������ﳬ���¶ȵ����¼���û��Ͼ���ṹ�еľ�����ͼ��ʾ��þԭ�Ӽ��γ����������������������µ��滹����һ��þԭ�ӣ�������ԭ��λ�������ڣ���û�����Ļ�ѧʽ�ɱ�ʾΪ
���㣺ԭ�ӹ���ӻ���ʽ���ӻ������ж�,ԭ�Ӻ�������Ų�,Ԫ�ص����ܡ��縺�Եĺ��弰Ӧ��,�����ļ���
ר�⣺ԭ�������ṹר��,��ѧ���뾧��ṹ
��������1��GaԪ�����ڵ�IIIA�壬��۵���Ϊ4s��4p���ӣ��ݴ���д��۵����Ų�ʽ��
��2��ͬһ����Ԫ�أ����һ����������ԭ����������������������ƣ�����IIA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
��3�����Ӿ�����۵����侧���ܳ����ȣ������������Ӱ뾶�ɷ��ȡ���������ɳ����ȣ�
��4�����ݼ۲���ӶԻ�������ȷ��BF3�ռ乹�ͼ�NF3������Nԭ�ӵ��ӻ�������ͣ����йµ��ӶԵ�ԭ�Ӻͺ��пչ����ԭ��֮�����γ���λ����
��5�����þ�̯��ȷ���û�����Ļ�ѧʽ��
��2��ͬһ����Ԫ�أ����һ����������ԭ����������������������ƣ�����IIA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
��3�����Ӿ�����۵����侧���ܳ����ȣ������������Ӱ뾶�ɷ��ȡ���������ɳ����ȣ�
��4�����ݼ۲���ӶԻ�������ȷ��BF3�ռ乹�ͼ�NF3������Nԭ�ӵ��ӻ�������ͣ����йµ��ӶԵ�ԭ�Ӻͺ��пչ����ԭ��֮�����γ���λ����
��5�����þ�̯��ȷ���û�����Ļ�ѧʽ��
���
�⣺��1��GaԪ�����ڵ�IIIA�壬��۵���Ϊ4s��4p���ӣ���۵����Ų�ʽΪ4s24p1���ʴ�Ϊ��4s24p1 ��
��2��ͬһ����Ԫ�أ����һ����������ԭ����������������������ƣ�����IIA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�����������Ԫ�ص�һ�����ܴ�С˳���Ǹƣ��أ��أ�
�ʴ�Ϊ���ƣ��أ��أ�
��3�����Ӿ�����۵����侧���ܳ����ȣ������������Ӱ뾶�ɷ��ȡ���������ɳ����ȣ������Ӱ뾶С�������ӣ����Է����Ƶľ����ܴ����廯�ƣ����۵�NaF��NaBr���ʴ�Ϊ���������ڷ����ƾ����ܴ����廯�ƣ�
��4�����ݼ۲���Ӷ�����֪��BF3������Bԭ�Ӽ۲���ӶԸ���=3+
����3-3��1��=3�Ҳ����µ��Ӷԣ�������ռ乹��Ϊƽ�������Σ�NF3������Nԭ�Ӽ۲���ӶԸ���=3+
����5-3��1��=4�Һ���һ���µ��Ӷԣ�����Nԭ�Ӳ���sp3�ӻ�����NH3?BF3��Bԭ�Ӻ��пչ����Nԭ�Ӻ��йµ��Ӷԣ����Զ������γ���λ����
�ʴ�Ϊ��ƽ���������Σ�sp3��B��
��5���þ����У�Bԭ�Ӹ���=6��Mgԭ�Ӹ���=2��
+12��
=3������Mgԭ�Ӻ�Bԭ�Ӹ���֮��=6��3=2��1�������仯ѧʽΪMgB2���ʴ�Ϊ��MgB2��
��2��ͬһ����Ԫ�أ����һ����������ԭ����������������������ƣ�����IIA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�����������Ԫ�ص�һ�����ܴ�С˳���Ǹƣ��أ��أ�
�ʴ�Ϊ���ƣ��أ��أ�
��3�����Ӿ�����۵����侧���ܳ����ȣ������������Ӱ뾶�ɷ��ȡ���������ɳ����ȣ������Ӱ뾶С�������ӣ����Է����Ƶľ����ܴ����廯�ƣ����۵�NaF��NaBr���ʴ�Ϊ���������ڷ����ƾ����ܴ����廯�ƣ�
��4�����ݼ۲���Ӷ�����֪��BF3������Bԭ�Ӽ۲���ӶԸ���=3+
| 1 |
| 2 |
| 1 |
| 2 |
�ʴ�Ϊ��ƽ���������Σ�sp3��B��
��5���þ����У�Bԭ�Ӹ���=6��Mgԭ�Ӹ���=2��
| 1 |
| 2 |
| 1 |
| 6 |
���������⿼�������ʽṹ�����ʣ��漰�����ļ��㡢���ռ乹�ͼ�ԭ���ӻ���ʽ���жϡ������ܵ�֪ʶ�㣬��Щ֪ʶ�㶼�Ǹ߿���Ƶ�㣬���þ�̯�����۲���ӶԻ������ۡ������������Ӿ����۵�Ĺ�ϵ��֪ʶ�����������ע�⣨5���ж����ϵ�ԭ�ӱ�6���������ö�����8��������Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ
�������ϲ����������ԭ��صĻ�ѧ��Ӧ�ǣ�������
| A��Al��OH��3��s��+NaOH��aq���TNaAlO2��aq��+2H2O��l����H��0 |
| B��CH3CH2OH��l��+3O2��g���T2CO2��g��+3H2O��l����H��0 |
| C��4Fe��OH��2��s��+2H2O��l��+O2��g���T4Fe��OH��3��s����H��0 |
| D��H2��g��+Cl2��g���T2HCl��g����H��0 |
������ͼװ�ã�����������ȷ���ǣ�������


| A��ͼ1װ�ÿɼ���С�մ��ƶ�����̼���� |
| B��ͼ1װ����ͭ��ϡ���ᷴӦ��һ���������� |
| C��ͼ3װ�õ���ƿ�г���NO2���壬�ձ���ʢˮ������Һ��ɳ���������ƿ |
| D��ͼ2װ�������ڸ���ռ�HCl���� |

 ������HBr����1��1�ӳɵIJ��������
������HBr����1��1�ӳɵIJ��������
 R���Ա�KMnO4��������Һ��������
R���Ա�KMnO4��������Һ�������� COOH���������R��ֱ���뱽�����ӵ�̼ԭ����û��CһH���������ױ������õ�
COOH���������R��ֱ���뱽�����ӵ�̼ԭ����û��CһH���������ױ������õ� COOH��
COOH�� COOH���칹�干��7�֣����е�3����
COOH���칹�干��7�֣����е�3���� CH2CH2CH2CH2CH3��
CH2CH2CH2CH2CH3�� ��
�� ��д������4�ֵĽṹ��ʽ��
��д������4�ֵĽṹ��ʽ�� ��1��ͼ1��һ�����׳�ҩ--ɽ����Ľṹ��ʽ����ȷ�������ʽΪ��
��1��ͼ1��һ�����׳�ҩ--ɽ����Ľṹ��ʽ����ȷ�������ʽΪ�� ��ͼ��ʾ����һ��ԭ���װ�ã�����
��ͼ��ʾ����һ��ԭ���װ�ã����� ��A��B��C��D��E����Ԫ�أ�A��B��C��D��E�ֱ����Ԫ�ط��ţ�������AԪ��ԭ�Ӻ���ֻ��һ�����ӣ�BԪ�صĻ�̬ԭ��s�Dz���ܵ�������p�Dz���ܵ�������1��CԪ�ص�ԭ�������������Ǵ�����������3����Dλ��B����һ���ڣ���ͬ����Ԫ���γɵļ������У�D�γɵļ����Ӱ뾶��С��E�Ļ�̬ԭ���е����Ų��������ܼ��ϣ����������ܼ���������������ͬ��
��A��B��C��D��E����Ԫ�أ�A��B��C��D��E�ֱ����Ԫ�ط��ţ�������AԪ��ԭ�Ӻ���ֻ��һ�����ӣ�BԪ�صĻ�̬ԭ��s�Dz���ܵ�������p�Dz���ܵ�������1��CԪ�ص�ԭ�������������Ǵ�����������3����Dλ��B����һ���ڣ���ͬ����Ԫ���γɵļ������У�D�γɵļ����Ӱ뾶��С��E�Ļ�̬ԭ���е����Ų��������ܼ��ϣ����������ܼ���������������ͬ��