��Ŀ����
8����������A ��NaH������Ҫ�Ļ�ԭ������ˮ��ǿ�ҷ�Ӧ��һ�������£�2.40g NaH������B��Ӧ����3.90g������A�� 2.24L��������ɱ�״������H2����֪����B��ʹʪ���ɫʯ����ֽ��������ش��������⣺
��1��A�Ļ�ѧʽ��NaNH2��
��2��NaH������B��Ӧ���ɻ�����A�Ļ�ѧ����ʽNaH+NH3=NaNH2+H2��
��3��A���������ᷢ����������ԭ��Ӧ�Ļ�ѧ����ʽNaNH2+2HCl=NaCl+NH4Cl��
��4���ڸ������⻯�ƣ�NaH���ɽ����Ȼ��ѣ�TiCl4����ԭ�ɽ����ѣ��÷�Ӧ�Ļ�ѧ����ʽΪ2NaH+TiCl4=Ti+2NaCl+2HCl��
��5��ijͬѧ��Ϊ��������B��ˮ��Һ���չ�ҵ������β���е�SO2��������Һ��ͨ�������ᾧ�ƵõĹ��弴Ϊ�������������Σ�ȡ�����ù����ˮ�ܽ⣬�ټ������BaCl2��Һ����������ɫ����������֤���õ��Ĺ���һ���Ǵ�����жϸ�ͬѧ������Ʊ����鴿�����ĺ����Բ�˵�����ɲ�������ͨ�������ᾧ�ƵõĹ���������������κ������εĻ������Եõ��Ĺ��岻һ���Ǵ���������������ᱵ�����ᱵ�Ļ���
���� ��֪����B��ʹʪ���ɫʯ����Һ������B�ǰ�����2.40gNaH�����ʵ���Ϊ0.1mol�Ͱ���B��Ӧ����3.90g������A��0.1molH2��Ϊ0.2g�����������غ㣬���뷴Ӧ�İ���������Ϊ1.7g����Ϊ0.1mol����������0.1molNaH+0.1molNH3=0.1molH2+A�����������غ���A��ѧʽΪNaNH2����Ħ������Ϊ39g/mol����3.90gNaNH2�����ʵ���Ϊ0.1mol���������⣬�ɴ˷������
��� �⣺��֪����B��ʹʪ���ɫʯ����Һ������B�ǰ�����2.40gNaH�����ʵ���Ϊ0.1mol�Ͱ���B��Ӧ����3.90g������A��0.1molH2��Ϊ0.2g�����������غ㣬���뷴Ӧ�İ���������Ϊ1.7g����Ϊ0.1mol����������0.1molNaH+0.1molNH3=0.1molH2+A�����������غ���A��ѧʽΪNaNH2����Ħ������Ϊ39g/mol����3.90gNaNH2�����ʵ���Ϊ0.1mol��
��1��A�Ļ�ѧʽ��NaNH2���ʴ�Ϊ��NaNH2��
��2��NaH������B��Ӧ���ɻ�����A�Ļ�ѧ����ʽ��NaH+NH3=NaNH2+H2���ʴ�Ϊ��NaH+NH3=NaNH2+H2��
��3��A���������ᷢ����������ԭ��Ӧ�Ļ�ѧ����ʽNaNH2+2HCl=NaCl+NH4Cl���ʴ�Ϊ��NaNH2+2HCl=NaCl+NH4Cl��
��4���ڸ����£�NaH���ɽ����Ȼ��ѣ�TiCl4����ԭ�ɽ����ѣ��÷�Ӧ�Ļ�ѧ����ʽΪ2NaH+TiCl4=Ti+2NaCl+2HCl���ʴ�Ϊ��2NaH+TiCl4=Ti+2NaCl+2HCl��
��5����Ϊ�������ξ��м�ǿ�Ļ�ԭ�ԣ�����Һ��ͨ�������ᾧ�ƵõĹ���������������κ������εĻ����ʴ�Ϊ����������ͨ�������ᾧ�ƵõĹ���������������κ������εĻ������Եõ��Ĺ��岻һ���Ǵ���������������ᱵ�����ᱵ�Ļ���
���� ���⿼��֪ʶ��϶࣬��ѧ���̵���д�������غ㶨�ɵ�Ӧ�á�������ԭ��Ӧ����ʽ����ƽ��Ԫ�ػ���������ʵȣ�����ƴ������Ŀ����Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�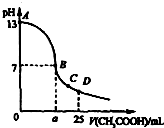 25��ʱ����25mL 0.1mol•L-1��NaOH��Һ�У���μ���0.2mol•L-1��CH3COOH��Һ����ҺpH�ı仯������ͼ��ʾ�����з����Ľ����У�����ȷ���ǣ�������
25��ʱ����25mL 0.1mol•L-1��NaOH��Һ�У���μ���0.2mol•L-1��CH3COOH��Һ����ҺpH�ı仯������ͼ��ʾ�����з����Ľ����У�����ȷ���ǣ�������| A�� | C��ʱc��CH3COO-����c��Na+����c��H+����c��OH-�� | |
| B�� | D��ʱc��CH3COO-��+c��CH3COOH��=2c��Na+�� | |
| C�� | ������A��B����һ�㣬��Һ�ж��У�c��Na+����c��CH3COO-����c��OH-����c��H+�� | |
| D�� | B������a=12.5ml |
| A�� | ���������ȡ����Ӧ�����ڹ��������²��ܽ��� | |
| B�� | ����ϩ�ۺϳɾ�����ϩ���ϵı����Ǽӳɷ�Ӧ | |
| C�� | �������ȡ����Ӧ�Ĵ���������FeBr3��Ҳ������Fe�� | |
| D�� | ���������ȡ��ȡ�ӳɷ�Ӧ��ȡ����Ӧ�ķ�����һ�� |
| A�� | CH3CH2CH2CH3 | B�� | CH3CH��CH3��2 | C�� | CH3C��CH3��3 | D�� | ��CH3��2CHCH2CH3 |
| A�� | nCH2�TCH2-��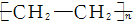 | B�� | CH2�TCH2+HCl-��CH3CH2Cl | ||
| C�� | 2CH3CH2OH+O2$��_{��}^{Cu}$2CH2CHO+2H2O | D�� | 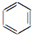 +Br2$\stackrel{FeBr_{3}}{��}$ +Br2$\stackrel{FeBr_{3}}{��}$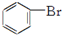 +HBr +HBr |
| A�� | ��ά�� | B�� | ������ | C�� | ����ϩ | D�� | ���� |
 ��
��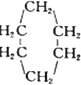 �ɱ�ʾΪ
�ɱ�ʾΪ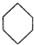 ��������ij�߾���ĺϳ�·�ߣ�������������⣺
��������ij�߾���ĺϳ�·�ߣ�������������⣺ $��_{��}^{NH}$
$��_{��}^{NH}$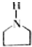 $��_{��}^{CH=CH}$A$\stackrel{��}{��}$
$��_{��}^{CH=CH}$A$\stackrel{��}{��}$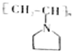
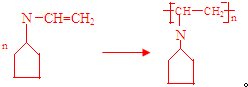 ��
��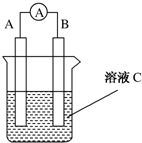 ��ͼ��ʾ��ԭ��ص�װ��ͼ��
��ͼ��ʾ��ԭ��ص�װ��ͼ�� Ϊ������������ش�
Ϊ������������ش�