��Ŀ����
16���������¼����л����CH4 ��CH3CH2OH ��
 �ܹ��� ��CH3COOH
�ܹ��� ��CH3COOH ��
 ��
�� ��
�� �����
������������������������ʰ�Ҫ��ش��������⣺
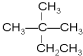
��1����Է�������Ϊ44�������Ľṹ��ʽΪCH3CH2CH3��
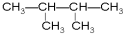
��2�������к���14����ԭ�ӵ������ķ���ʽ��C6H14��
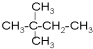
��3����ۻ�Ϊͬ���칹����Ǣߣ�����ţ���
��4������������ζ��������ȡ�����л�����������������������Һ�巢��һȡ����Ӧ�Ļ�ѧ����ʽ
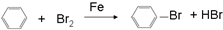 ��
����5���л�����ڼ��������º�CuO��Ӧ�Ļ�ѧ����ʽ
 ��
����6���л���ݺ͢���һ�������·�����Ӧ�Ļ�ѧ����ʽ��
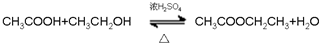 ��
��
���� ��1������������ͨʽCnH2n+2��������Է�������Ϊ44��ʽ���㣻
��2������������ͨʽCnH2n+2����14����ԭ�Ӵ���ͨʽ���㣻
��3��ͬ���칹����ָ����ʽ��ͬ�����ṹ��ͬ�Ļ����
��4������������ζ��������ȡ�������л�����Һ�巢��ȡ����Ӧ����Ϊ���������嵥�ʷ�Ӧ�����屽��
��5���Ҵ���CuO��Ӧ������ȩ��Cu��ˮ��
��6���������Ҵ�����������Ӧ��������������ˮ��
��� �⣺��1��������ͨʽΪ��CnH2n+2����Է�������Ϊ44����������12n+2n+2=44������n=3���������ķ���ʽΪC3H8��Ϊ����飬�ṹ��ʽΪCH3CH2CH3��
�ʴ�Ϊ��CH3CH2CH3��
��2�������ķ����к���14����ԭ�ӣ�������ͨʽΪ��CnH2n+2����2n+2=14������n=6���������ķ���ʽΪC6H14��Ϊ���飬���ۢߢ࣬
�ʴ�Ϊ��C6H14��
��3���ۢ߷���ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壬�����Ϊͬһ�����ʣ�
�ʴ�Ϊ���ߣ�
��4������������ζ��������ȡ�����л���Ϊ������������������������Һ�巢��һȡ����Ӧ�Ļ�ѧ����ʽΪ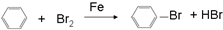 ��
��
�ʴ�Ϊ��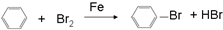 ��
��
��5���Ҵ���CuO��Ӧ������ȩ��Cu��ˮ����ӦΪ ��
��
�ʴ�Ϊ�� ��
��
��6���������Ҵ�����������Ӧ�������ǻ������⣬��������������ˮ���÷�ӦΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л���Ľṹ�����ʣ����չ����������ʵĹ�ϵ���л���ӦΪ���Ĺؼ������ط�����Ӧ�������Ŀ��飬�ۺ��Խ�ǿ����Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�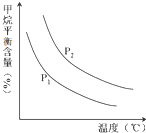 �ϳɰ������Ĵ����������˹��̵�����Ҫ;�������о�������ȷ������ָ�����ϳɰ���Ӧ��ƽ�ⳣ��Kֵ���¶ȵĹ�ϵ���£�
�ϳɰ������Ĵ����������˹��̵�����Ҫ;�������о�������ȷ������ָ�����ϳɰ���Ӧ��ƽ�ⳣ��Kֵ���¶ȵĹ�ϵ���£�| �� �ȣ��棩 | 360 | 440 | 520 |
| Kֵ | 0.036 | 0.010 | 0.0038 |
�����ϱ����ݿ�֪�÷�ӦΪ���ȷ�Ӧ�����������¶����ߣ���Ӧ��ƽ�ⳣ��K��С��
�������ϣ�Ϊ������ƽ��ʱH2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��ad��������ţ�
a������ѹǿ b��ʹ�ú��ʵĴ���
c�������¶� d����ʱ����������е�NH3
��2��ԭ����H2��ͨ����Ӧ CH4��g��+H2O ��g���TCO��g��+3H2��g�� ��ȡ����֪�÷�Ӧ�У�����ʼ������е� $\frac{n��{H}_{2}O��}{n��C{H}_{4}��}$ �㶨ʱ���¶ȡ�ѹǿ��ƽ������CH4������Ӱ����ͼ��ʾ��
��ͼ�У��������߱�ʾѹǿ�Ĺ�ϵ�ǣ�P1��P2�����������=����������
�ڸ÷�ӦΪ���ȷ�Ӧ������ȡ����ȡ�����
��3��ԭ����H2����ͨ����ӦCO��g��+H2O��g���TCO2 ��g��+H2��g�� ��ȡ��
��T��ʱ�����ݻ��̶�Ϊ5L�������г���1molˮ������1mol CO����Ӧ��ƽ����CO��Ũ��Ϊ0.08mol•L-1����ƽ��ʱCO��ת����Ϊ60%���÷�Ӧƽ�ⳣ���ı���ʽΪ2.25��
�ڱ����¶���ΪT�棬�ı�ˮ������CO�ij�ʼ���ʵ���֮�ȣ������������з�Ӧ�����������ܹ�˵����ϵ����ƽ��״̬����cd������ţ���
a��������ѹǿ����ʱ��ı�
b�����������ܶȲ���ʱ��ı�
c����λʱ��������a mol CO2��ͬʱ����a mol H2
d���������n ��CO����n ��H2O����n ��CO2����n ��H2��=1��1��1��1��
| A�� | H2��D2��Ϊͬλ�� | B�� | 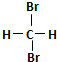 �� �� 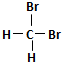 ��Ϊͬ���칹�� ��Ϊͬ���칹�� | ||
| C�� | ��������춡����ͬϵ�� | D�� | 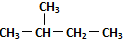 �� �� 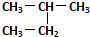 ��ͬһ������ ��ͬһ������ |
| �� | �� | �� |
| ���� | �ƹ� | ��� |
| HOCH2COOH | C3H4O5 | 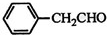 |
��
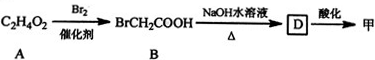
��1mol������NaHCO3 �����ʵ����Ǽ�2��
��
 $\stackrel{Fe��HCl}{��}$
$\stackrel{Fe��HCl}{��}$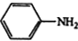 $��_{��2��H_{2}O��H+����}^{��1��NaNO_{2}��HCl}$
$��_{��2��H_{2}O��H+����}^{��1��NaNO_{2}��HCl}$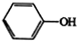
�ش��������⣺
��1��������֪�ٵõ���
�ټ��к��в����ͼ��Ĺ���������Ϊ�Ȼ���
��A��BΪȡ����Ӧ��A�Ľṹ��ʽΪCH3COOH��
��B��D�Ļ�ѧ����ʽΪBrCH2COOH+2NaOH$��_{��}^{H_{2}O}$HOCH2COONa+NaBr+H2O��
��2������һ��������������״�����л��߷��ӻ�ѧ��Ļ�ѧ����ʽΪn HOOCCH��OH��COOH$\stackrel{һ������}{��}$
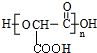 +��n-1��H2O��
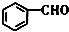
+��n-1��H2O����3���ɱ�������;���ɵ�һ����Ҫ��ҽҩ�������м���J�����ַ�Ӧ������ȥ����
��$��_{��������}^{H_{2}}$
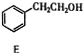 $\stackrel{������}{��}$
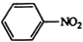
$\stackrel{������}{��}$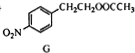 $��_{��2��H+����}^{��1��Fe��HCl}$$\stackrel{{C}_{8}{H}_{11}NO}{H}$$��_{��2��H_{2}O��H+����}^{��1��NaNO_{2}��HCl}$J
$��_{��2��H+����}^{��1��Fe��HCl}$$\stackrel{{C}_{8}{H}_{11}NO}{H}$$��_{��2��H_{2}O��H+����}^{��1��NaNO_{2}��HCl}$J���û�ѧ������ȥE�в�����������������ʱE�ͱ���Һ̬������������ˮ�е��ܽ⣩����1�������Լ�������Ϊ����������ͭ��������Һ����2��3 �������ֱ��ǹ��ˡ���Һ��
�ھ�E��G��H�����Ĺ��������ǻ������Ա����л��������д��ں��ֹ����ŵ������Ǻ�������ǣ�
��J��ͬ���칹�����ں˴Ź�����������ʾΪ����壬�������Ϊ3��2����״�Ҳ�����֧�����칹�干��8�֣����������칹��������ij�칹��L�еĹ����Ŷ�����H2 �����ӳɷ�Ӧ����L�Ľṹ��ʽΪCH3CH2COC��CCOCH2CH3��CH3COCH2C��CCH2COCH3��ֻдһ�֣���
| A�� | �ںϳɰ��Ĺ�ҵ�����У�ʹ�ýϸ��¶���������߲��� | |
| B�� | �ںϳɰ��Ĺ�ҵ�����У���ѹ��������߰��IJ��� | |
| C�� | ľ̿�������O2��Ӧ�����ʸ��� | |
| D�� | ��H2��g����I2��g����HI������ɵ�ƽ����ϵ��ѹ����ɫ���� |
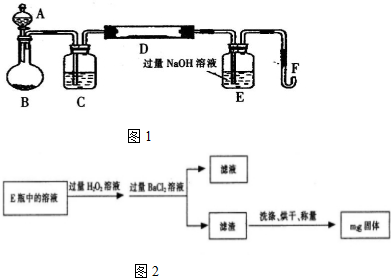
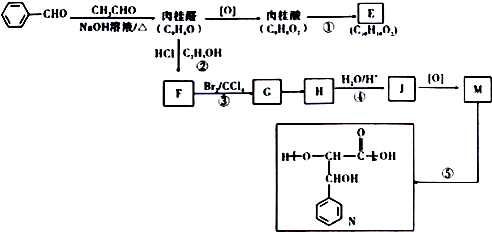
 �������DZ���ȩ��
�������DZ���ȩ��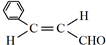 �������������18��ԭ�ӹ�ƽ�森
�������������18��ԭ�ӹ�ƽ�森 ��
��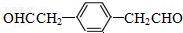 ��
��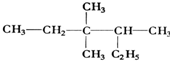 3��3��4-��������
3��3��4-�������� 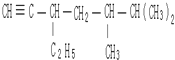 5��6-����-3-�һ�-1-��Ȳ
5��6-����-3-�һ�-1-��Ȳ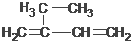 2-�һ�-1��3-����ϩ
2-�һ�-1��3-����ϩ 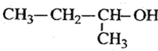 2-����
2-����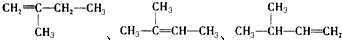 ��
��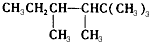 ����A�ǵ�ϩ���������ӳɺ�IJ����õ�ϩ��������5�ֽṹ��
����A�ǵ�ϩ���������ӳɺ�IJ����õ�ϩ��������5�ֽṹ��