��Ŀ����
 ij��A�����ϣԪ�ط���ʵ����̼����������Ϊ85.71%������������ͼ��ʾ���������ӷ������ʺɱ�Ϊ84�������ĺ˴Ź���������ͼ��ʾ��������ױ���������û��̼̼˫������֪ϩ�����������÷�����Ӧ��
ij��A�����ϣԪ�ط���ʵ����̼����������Ϊ85.71%������������ͼ��ʾ���������ӷ������ʺɱ�Ϊ84�������ĺ˴Ź���������ͼ��ʾ��������ױ���������û��̼̼˫������֪ϩ�����������÷�����Ӧ��CH3CH=CHCH3
| ��O3 |
| ��H2O |
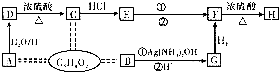
A������ת����ϵ���Ժϳɸ߷��ӻ�����I������B������ֻ����һ����ԭ�ӣ�F��A���ʽ��ͬ����F������״�������һ�֣�

��ش��������⣺
��1��д���������ʵĽṹ��ʽ��A
��2��д�����з�Ӧ���ͣ�A��B
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��G��H��NaOH������
��4��A����״ͬ���칹���У�����˳���칹��A��ͬ���칹����
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
��������A��̼����������Ϊ85.71%������Ԫ�ص���������Ϊ1-85.71%=14.29%����N��C����N��H��=
��
=1��2���ʸ��������ʽΪCH2���������ӷ���ʺɱ�Ϊ84����������Է�������Ϊ84�������Ϊ��CH2��n����14n=84�����n=6���ʸ÷���ʽΪC6H12���ɸ����ĺ˴Ź�������֪���˴Ź�������ֻ��1���壬������ֻ��1��Hԭ�ӣ�������ױ���������û��̼̼˫������AΪ ������������ȡ����Ӧ����B��B������ֻ����һ����ԭ�ӣ���BΪ
������������ȡ����Ӧ����B��B������ֻ����һ����ԭ�ӣ���BΪ ��B������ȥ��Ӧ����CΪ
��B������ȥ��Ӧ����CΪ ��C������Ϣ�г�����������DΪOHC��CH2��4CHO��D����������Ӧ����EΪHOOC��CH2��4COOH��F��A���ʽ��ͬ����F������״�������һ�֣���FΪCH2=CH2�����巢���ӳɷ�Ӧ����GΪBrCH2CH2Br��G����ˮ�ⷴӦ����HΪHOCH2CH2OH��E��H�������۷�Ӧ���ɸ߾���HΪ
��C������Ϣ�г�����������DΪOHC��CH2��4CHO��D����������Ӧ����EΪHOOC��CH2��4COOH��F��A���ʽ��ͬ����F������״�������һ�֣���FΪCH2=CH2�����巢���ӳɷ�Ӧ����GΪBrCH2CH2Br��G����ˮ�ⷴӦ����HΪHOCH2CH2OH��E��H�������۷�Ӧ���ɸ߾���HΪ ���ݴ˽��
���ݴ˽��
| 85.71% |
| 12 |
| 14.29% |
| 1 |
 ������������ȡ����Ӧ����B��B������ֻ����һ����ԭ�ӣ���BΪ
������������ȡ����Ӧ����B��B������ֻ����һ����ԭ�ӣ���BΪ ��B������ȥ��Ӧ����CΪ
��B������ȥ��Ӧ����CΪ ��C������Ϣ�г�����������DΪOHC��CH2��4CHO��D����������Ӧ����EΪHOOC��CH2��4COOH��F��A���ʽ��ͬ����F������״�������һ�֣���FΪCH2=CH2�����巢���ӳɷ�Ӧ����GΪBrCH2CH2Br��G����ˮ�ⷴӦ����HΪHOCH2CH2OH��E��H�������۷�Ӧ���ɸ߾���HΪ
��C������Ϣ�г�����������DΪOHC��CH2��4CHO��D����������Ӧ����EΪHOOC��CH2��4COOH��F��A���ʽ��ͬ����F������״�������һ�֣���FΪCH2=CH2�����巢���ӳɷ�Ӧ����GΪBrCH2CH2Br��G����ˮ�ⷴӦ����HΪHOCH2CH2OH��E��H�������۷�Ӧ���ɸ߾���HΪ ���ݴ˽��
���ݴ˽�����
�⣺��A��̼����������Ϊ85.71%������Ԫ�ص���������Ϊ1-85.71%=14.29%����N��C����N��H��=
��
=1��2���ʸ��������ʽΪCH2���������ӷ���ʺɱ�Ϊ84����������Է�������Ϊ84�������Ϊ��CH2��n����14n=84�����n=6���ʸ÷���ʽΪC6H12���ɸ����ĺ˴Ź�������֪���˴Ź�������ֻ��1���壬������ֻ��1��Hԭ�ӣ�������ױ���������û��̼̼˫������AΪ ������������ȡ����Ӧ����B��B������ֻ����һ����ԭ�ӣ���BΪ
������������ȡ����Ӧ����B��B������ֻ����һ����ԭ�ӣ���BΪ ��B������ȥ��Ӧ����CΪ
��B������ȥ��Ӧ����CΪ ��C������Ϣ�г�����������DΪOHC��CH2��4CHO��D����������Ӧ����EΪHOOC��CH2��4COOH��F��A���ʽ��ͬ����F������״�������һ�֣���FΪCH2=CH2��F���巢���ӳɷ�Ӧ����GΪBrCH2CH2Br��G����ˮ�ⷴӦ����HΪHOCH2CH2OH��E��H�������۷�Ӧ���ɸ߾���HΪ
��C������Ϣ�г�����������DΪOHC��CH2��4CHO��D����������Ӧ����EΪHOOC��CH2��4COOH��F��A���ʽ��ͬ����F������״�������һ�֣���FΪCH2=CH2��F���巢���ӳɷ�Ӧ����GΪBrCH2CH2Br��G����ˮ�ⷴӦ����HΪHOCH2CH2OH��E��H�������۷�Ӧ���ɸ߾���HΪ ��
��
��1��������������֪��AΪ ��CΪ
��CΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��
��2��������������֪��A��B����ȡ����Ӧ��D��E����������Ӧ��F��G���ڼӳɷ�Ӧ���ʴ�Ϊ��ȡ����Ӧ��������Ӧ���ӳɷ�Ӧ��
��3��G��H��NaOH��������Ӧ����ʽΪ��CH2BrCH2Br+2NaOH
CH2OHCH2OH+2NaBr��
E+H��I�ķ�Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ��CH2BrCH2Br+2NaOH
CH2OHCH2OH+2NaBr�� ��
��
��4��A�� ������״ͬ���칹���У�����˳���칹��A��ͬ���칹���У�CH3CH2CH=CHCH2CH3��CH3CH=CHCH2CH2CH3��CH3CH=CHCH��CH3��2��CH3CH=C��CH3��C2H5���ʴ�Ϊ��4��
������״ͬ���칹���У�����˳���칹��A��ͬ���칹���У�CH3CH2CH=CHCH2CH3��CH3CH=CHCH2CH2CH3��CH3CH=CHCH��CH3��2��CH3CH=C��CH3��C2H5���ʴ�Ϊ��4��
| 85.71% |
| 12 |
| 14.29% |
| 1 |
 ������������ȡ����Ӧ����B��B������ֻ����һ����ԭ�ӣ���BΪ
������������ȡ����Ӧ����B��B������ֻ����һ����ԭ�ӣ���BΪ ��B������ȥ��Ӧ����CΪ
��B������ȥ��Ӧ����CΪ ��C������Ϣ�г�����������DΪOHC��CH2��4CHO��D����������Ӧ����EΪHOOC��CH2��4COOH��F��A���ʽ��ͬ����F������״�������һ�֣���FΪCH2=CH2��F���巢���ӳɷ�Ӧ����GΪBrCH2CH2Br��G����ˮ�ⷴӦ����HΪHOCH2CH2OH��E��H�������۷�Ӧ���ɸ߾���HΪ
��C������Ϣ�г�����������DΪOHC��CH2��4CHO��D����������Ӧ����EΪHOOC��CH2��4COOH��F��A���ʽ��ͬ����F������״�������һ�֣���FΪCH2=CH2��F���巢���ӳɷ�Ӧ����GΪBrCH2CH2Br��G����ˮ�ⷴӦ����HΪHOCH2CH2OH��E��H�������۷�Ӧ���ɸ߾���HΪ ��
����1��������������֪��AΪ
 ��CΪ
��CΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
����2��������������֪��A��B����ȡ����Ӧ��D��E����������Ӧ��F��G���ڼӳɷ�Ӧ���ʴ�Ϊ��ȡ����Ӧ��������Ӧ���ӳɷ�Ӧ��
��3��G��H��NaOH��������Ӧ����ʽΪ��CH2BrCH2Br+2NaOH
| ˮ |
| �� |
E+H��I�ķ�Ӧ����ʽΪ��
 ��
���ʴ�Ϊ��CH2BrCH2Br+2NaOH
| ˮ |
| �� |
 ��
����4��A��
 ������״ͬ���칹���У�����˳���칹��A��ͬ���칹���У�CH3CH2CH=CHCH2CH3��CH3CH=CHCH2CH2CH3��CH3CH=CHCH��CH3��2��CH3CH=C��CH3��C2H5���ʴ�Ϊ��4��
������״ͬ���칹���У�����˳���칹��A��ͬ���칹���У�CH3CH2CH=CHCH2CH3��CH3CH=CHCH2CH2CH3��CH3CH=CHCH��CH3��2��CH3CH=C��CH3��C2H5���ʴ�Ϊ��4��
���������⿼���л����ƶϡ��л���Ӧ���͡�ͬ���칹����д�ȣ�������A�и�Ԫ�ص�������������ͼ֪ʶ����ȷ��A�Ľṹ�ǹؼ����ٽ��ת����ϵ�ƶϣ��Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
����H++OH-=H2O����ʾ�Ļ�ѧ��Ӧ�ǣ�������
| A��������ͭ��ϡ���ᷴӦ |
| B������������Һ����ϡ������ |
| C������������Һ�����ᷴӦ |
| D�����������������Һ |
������������ȷ���ǣ�������
| A����ʵ�������������Һ����ڴ��������Լ�ƿ�� |
| B���ᳫ���ǹ���ʱ�������ϴ�����Ϊ�˷�ֹ��ɫ��Ⱦ |
| C����������CCl2F2�����ƻ���������������¡�����ЧӦ�� |
| D��Ϊ��ֹ����е��ؽ�������Ⱦ������ˮ�壬Ӧ���������ϵ�ص��ۺ����ü��� |
����������ȷ���ǣ�������
| A��NaCl��Һ�ڵ����������µ����Na+ |
| B�����ӻ�����һ���ǵ���� |
| C����ˮ�������ԣ���NH3��������� |
| D������ˮ����������ӵĻ����ﶼ���� |
 �ס������ص缫���϶���������̼������ش��������⣺
�ס������ص缫���϶���������̼������ش��������⣺