��Ŀ����
16�� ʵ����ijŨ������Լ�ƿ�ϵı�ǩ����ͼ��ʾ�����ݱ�ǩ�ϵ��й����ݻش��������⣺
ʵ����ijŨ������Լ�ƿ�ϵı�ǩ����ͼ��ʾ�����ݱ�ǩ�ϵ��й����ݻش��������⣺��1�����Լ��� H2SO4�����ʵ���Ũ��Ϊ18.4mol•L-1��
��2��ijͬѧ������920mL���ʵ���Ũ��Ϊ0.30mol•L-1��ϡ���ᣬ����ѡ������Ϊ1000mL������ƿ��Ȼ�����ۼ�����Ҫ��ȡ16.3mL����Ũ���ᣨ����������С�����һλ����
��3�����и���ʵ��������ж���ȷ����D����д��ĸ����
A������0.1mol/L CuSO4��Һ100mL�������CuSO4•5H2O 1.6g
B������ƽ���������и���һ�Ű�ֽ�ɽ�NaOH����������̰�ֽ�ϳ���
C������Ͳ���Ծ�ȷ��ȡ25.03mLij��Һ��
D����Ҫ235mL 0.9mol/L NaCl��Һ��һ����250mL����ƿ��������
E�������ƺ�һ�����ʵ���Ũ�ȵ���Һ��ע�����ˮϴ�����Լ�ƿ�У�Ũ�Ȳ���Ӱ�죮
��4�����������ʹ������Һ�����ʵ���Ũ��ƫ�͵���CDE������ţ���
A������ƿ������ˮϴ����δ���ڱڸ������������
B���ռ����ձ���պ���ȫ�ܽ⣬��������Һת�Ƶ�����ƿ��
C������ʱ�����ӿ̶���
D��ҡ�Ⱦ��ú���Һ��δ���̶��ߣ���������ˮ���̶���
E�����ܽ������������Һ�彦���ձ��⣮
���� ��1������C=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ�
��2������������Һ���ѡ������ƿ���������Һϡ���������ʵ����ʵ������������ҪŨ����������
��3��A������m=CVM������Ҫ���ʵ�������
B��������ʴ��ҩƷ��Ӧ����С�ձ����߳���ƿ �У�
C��������Ͳ��ȷ���жϣ�
D������������Һ����ͳ�������ƿ����жϣ�
E��������Һϡ���ɽ��
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1��Ũ�������ʵ���Ũ��C=$\frac{1000��1.84��98%}{98}$=18.4mol/L��
�ʴ�Ϊ��18.4��
��2������920mL���ʵ���Ũ��Ϊ0.30mol•L-1��ϡ���ᣬû��920mL����ƿ��Ӧѡ��1000mL����ƿ������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ�������ã�18.4mol/L��V=0.30mol•L-1��1000mL�����V=16.3mL��
�ʴ�Ϊ��1000�� 16.3��
��3��A������0.1mol/L CuSO4��Һ100mL�������CuSO4•5H2O ����m=0.1mol/L��0.1L��250g/mol=2.5g����A����
B���������ƾ��и�ʴ�ԣ�Ӧ����С�ձ����߳���ƿ�н��У���B����
C����Ͳ��ȷ��Ϊ0.1mL�����Զ���ʱС�����Ӧ����1λС������C����
D����Ҫ235mL 0.9mol/L NaCl��Һ��ʵ����û��235mL����ƿ��Ӧѡ��250mL����ƿ����D��ȷ��
E�������ƺ�һ�����ʵ���Ũ�ȵ���Һ��ע�����ˮϴ�����Լ�ƿ�У�������Һ��ϡ�ͣ���ҺŨ��ƫС����E����
��ѡ��D��
��4��A������ƿ������ˮϴ����δ���ڱڸ�����������ƣ������ʵ����ʵ�������Һ������������Ӱ�죬��ҺŨ�Ȳ��䣬��A��ѡ��
B���ռ����ձ���պ���ȫ�ܽ⣬��������Һת�Ƶ�����ƿ�У���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���B��ѡ��
C������ʱ�����ӿ̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���Cѡ��
D��ҡ�Ⱦ��ú���Һ��δ���̶��ߣ���������ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���Dѡ��
E�����ܽ������������Һ�彦���ձ��⣬�������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���Eѡ��
��ѡ��CDE��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������������ǽ���ؼ���ע���������ķ����ͼ��ɣ���Ŀ�ѶȲ���

| ѡ�� | ʵ����� | ���� | ���� |
| A | ��ʢ��1mLŨ������Թ��м���5mL 0.1mol•L-1 �� K2Cr2O7��Һ | ��Һ��ɫ���� | ����������Ũ�ȣ�ƽ��Cr2O72-����ɫ��+H2O?2CrO42- ����ɫ��+2H+������ �� |
| B | ��Mg��OH��2����Һ�м������� �Ȼ�茶��� | �����ܽ⣬��Һ����� | ˵����ӦMg��OH��2+2NH4+?Mg2++2NH3•H2O���п����� |
| C | ����Ƥ����п�������ֹκۺ���� �ڱ���ʳ��ˮ�У�һ��ʱ�����뼸��K3[Fe��CN��6]��Һ | ���������� | �ù���δ����ԭ��ط�Ӧ |
| D | ��ú¯�����ȵ�ú̿��������ˮ | ��������ɫ�� �棬ú̿ȼ�ո��� | ������ˮ��ʹú̿ȼ�շų���������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ������Ϊ53��������Ϊ78�ĵ�ԭ�ӣ�53131I | |
| B�� | CH4���ӵı���ģ�ͣ� | |
| C�� | Na+ �Ľṹʾ��ͼ�� | |
| D�� | NaOH�ĵ��뷽��ʽ��NaOH?Na++OH- |
| A�� | �Ͻ�¶�����ũ����ո� | |
| B�� | ��ǿֲ�����֣������̻���� | |
| C�� | ������������ʹ����Ȼ��������Ϊȼ�� | |
| D�� | ���������мӴ�ú̿��ʯ�͵�ȼ�ϵ�ʹ���� |
I��������Һ����NaOH��ҺΪ����
��1��ʵ����Ҫ0.5mol/L NaOH��Һ245mL����������ҪNaOH����5.0g��
��2������NaOH��Һ�����У�һ����Ҫ���������е���д�����ı�ţ���ͼ21
II���ⶨNaOH��Һ��ϡ H2SO4��Ӧ���к��� ȡ50mLNaOH��Һ��30mL H2SO4��Һ����ʵ�飬������������
| ��ʼ�¶�/�� | ��ֹ�¶�/�� | �¶Ȳ�/�� | |||
| H2SO4��Һ | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.0 | 24.5 | 24.75 | 29.3 | 4.55 |
| 2 | 24.5 | 24.2 | 24.35 | 28.3 | 3.95 |
| 3 | 25.0 | 24.5 | 24.75 | 28.7 | 3.95 |
��4������ʵ���������һ57.3kJ/mol����ƫ���ԭ�������ACD������ţ�
A��ʵ��װ�ñ��¡�����Ч����
B��ȡNaOH��Һ���ʱ���Ӷ���
C���ֶ�ν�NaOH��Һ����ʢ�������С�ձ���
D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2S04��Һ���¶�
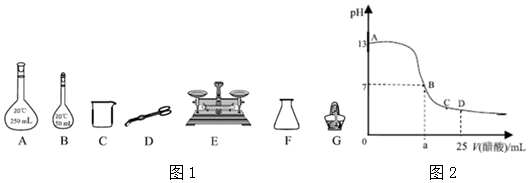
III����25mLNaOH��Һ����μ���0.2mol/LCH3COOH��Һ���ζ�������ͼ��ʾ��
��5��д��NaOH��Һ��CH3COOH��Һ��Ӧ�����ӷ���ʽCH3COOH+OH-=CH3COO-+H2O��
��6����NaOH��Һ�����ʵ���Ũ��Ϊ0.1mol/L��
��7�����μ�CH3COOH��Һ��D��ʱ�����û���� Һ�и�����Ũ�ȵĴ�СΪc��CH3COO-����c��Na+����c��H+����c��OH-����
| A�� | ���������������������ֱ���ȫȼ�գ����߷ų��������� | |
| B�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ | |
| C�� | ��101 kPaʱ��2 g H2��ȫȼ������Һ̬ˮ���ų�285.8 kJ����������ȼ�յ��Ȼ�ѧ����ʽΪ��2H2��g��+O2��g���T2H2O��l����H=+285.8 kJ/mol | |
| D�� | ϡ��Һ�У�H+��aq��+OH-��aq���TH2O��l����H=-57.3 kJ/mol��ŨH2SO4�뺬1 mol NaOH����Һ��ϣ��ų�����������57.3 kJ/mol |