��Ŀ����
��ij��ʱ����־������һ����Ϊƻ���ף�ACV����Ũ�����϶��������ҵ��أ���ƻ�������������ϵ���Ҫ�������ʣ��Դ���Ļ�ѧ�������£�
a��ȼ�յIJ�����CO2��H2O��̼�������������ֱ�Ϊ��C-35.82%��H-4.48%��
b��1.00mmol������������NaHCO3��Ӧ���ų�44.8mLCO2������������Na��Ӧ�ų�33.6mLH2�����������������Ϊ��״������
c���ṹ�����������÷����д������ֻ�ѧ������ͬ��̼ԭ��
�Իش��������⣺
��1��ƻ����Ļ�ѧʽΪ ���ṹ��ʽΪ
��2��д��������һ�������·�������ȥһ����ˮ�IJ���Ŀ��ܽṹ��ʽ �� ��д�����ּ��ɣ�
a��ȼ�յIJ�����CO2��H2O��̼�������������ֱ�Ϊ��C-35.82%��H-4.48%��
b��1.00mmol������������NaHCO3��Ӧ���ų�44.8mLCO2������������Na��Ӧ�ų�33.6mLH2�����������������Ϊ��״������
c���ṹ�����������÷����д������ֻ�ѧ������ͬ��̼ԭ��
�Իش��������⣺
��1��ƻ����Ļ�ѧʽΪ
��2��д��������һ�������·�������ȥһ����ˮ�IJ���Ŀ��ܽṹ��ʽ
���㣺�й��л������ʽȷ���ļ���
ר�⣺�������������ȼ�չ���
������a��������C��H���������ֱ�Ϊw��C��=35.82%��w��H��=4.48%����O����������Ϊ��1-35.82%-4.48%=59.7%��
b.1mol������������NaHCO3��Ӧ�ų�44.8L CO2��˵�������к���2��-COOH����������Na��Ӧ�ų������33.6L H2�����ʵ���Ϊ1.5mol��˵�������к���2��-COOH��1��-OH�����л�������к���5��Oԭ�ӣ����л������Է�����Ϊ��
=134����C������
=4����H������
=6�������ʽΪC4H6O5��
c���÷����д������ֻ�ѧ������ͬ��̼ԭ�ӣ���ÿ��̼���Ӳ�ͬ�Ļ��ţ���ԭ�Ӵ������ֲ�ͬ�Ļ�ѧ������˵�������ֲ�ͬ��Hԭ�ӣ��ṹӦΪ ���Դ˽����⣮
���Դ˽����⣮
b.1mol������������NaHCO3��Ӧ�ų�44.8L CO2��˵�������к���2��-COOH����������Na��Ӧ�ų������33.6L H2�����ʵ���Ϊ1.5mol��˵�������к���2��-COOH��1��-OH�����л�������к���5��Oԭ�ӣ����л������Է�����Ϊ��
| 16��5 |
| 0.597 |
| 134��35.83% |
| 12 |
| 134��4.48% |
| 1 |
c���÷����д������ֻ�ѧ������ͬ��̼ԭ�ӣ���ÿ��̼���Ӳ�ͬ�Ļ��ţ���ԭ�Ӵ������ֲ�ͬ�Ļ�ѧ������˵�������ֲ�ͬ��Hԭ�ӣ��ṹӦΪ
 ���Դ˽����⣮
���Դ˽����⣮���
�⣺��1��a��������C��H���������ֱ�Ϊw��C��=35.82%��w��H��=4.48%����O����������Ϊ��1-35.82%-4.48%=59.7%��
b.1mol������������NaHCO3��Ӧ�ų�44.8L CO2��˵�������к���2��-COOH����������Na��Ӧ�ų������33.6L H2�����ʵ���Ϊ1.5mol��˵�������к���2��-COOH��1��-OH�����л�������к���5��Oԭ�ӣ����л������Է�����Ϊ��
=134����C������
=4����H������
=6�������ʽΪC4H6O5��
c���÷����д������ֻ�ѧ������ͬ��̼ԭ�ӣ���ÿ��̼���Ӳ�ͬ�Ļ��ţ���ԭ�Ӵ������ֲ�ͬ�Ļ�ѧ������˵�������ֲ�ͬ��Hԭ�ӣ��ṹӦΪ ��
��
�ʴ�Ϊ��C4H6O5�� ��
��
��2�����������١�������������ͬ���칹�壬�����к���2��-COOH��1��-OH������������µ�ͬ���칹���У� ��
��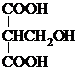 ��
��
�ʴ�Ϊ�� ��
��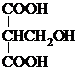 ��
��
b.1mol������������NaHCO3��Ӧ�ų�44.8L CO2��˵�������к���2��-COOH����������Na��Ӧ�ų������33.6L H2�����ʵ���Ϊ1.5mol��˵�������к���2��-COOH��1��-OH�����л�������к���5��Oԭ�ӣ����л������Է�����Ϊ��
| 16��5 |
| 0.597 |
| 134��35.83% |
| 12 |
| 134��4.48% |
| 1 |
c���÷����д������ֻ�ѧ������ͬ��̼ԭ�ӣ���ÿ��̼���Ӳ�ͬ�Ļ��ţ���ԭ�Ӵ������ֲ�ͬ�Ļ�ѧ������˵�������ֲ�ͬ��Hԭ�ӣ��ṹӦΪ
 ��
���ʴ�Ϊ��C4H6O5��
 ��
����2�����������١�������������ͬ���칹�壬�����к���2��-COOH��1��-OH������������µ�ͬ���칹���У�
 ��
��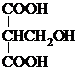 ��
���ʴ�Ϊ��
 ��
��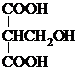 ��
��
���������⿼���л�����ƶϣ���Ŀ�Ѷ��еȣ�ע�������ĿҪ�������Է������������ݽṹ�ص���д�ṹ��ʽ������ͬ���칹�����д������

��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
�����Ŀ
���и������ӣ���ָ��������һ���ܴ���������ǣ�������
| A���������þ�ܲ���H2����Һ�У�Na+��Fe3+��SO42-��NO3- |
| B��c��OH-��=1��10-3mol/L����Һ�У�K+��SO42-��Cl-��CO32- |
| C����ʹpH��ֽ�����ɫ����Һ�У�S2-��SO32-��Na+��SO42- |
| D����ˮ�������c��OH-��=1��10-13mol/L����Һ�У�Fe2+��NH4+��ClO-��Cl- |
�������ӷ���ʽ��������ʵ�������ȷ���ǣ�������
A��Ư����Һ�ڿ�����ʧЧ��ClO-+CCO2+H2O=HClO+HC
| ||
B��������KMnO4��Һ�м���H2O2����ɫ��ȥ��2Mn
| ||
C����NaHSO4��Һ�еμ�Ba��OH��2��Һ�����ԣ�H++S
| ||
| D����Ũ������MnO2��Ӧ��ȡ����������MnO2+2H++2Cl-�TMn2++Cl2��+2H2O |
��NA���������ӵ�����������˵����ȷ���ǣ�������
| A���ڳ��³�ѹ�£�2.24 L CO2�к��е�ԭ����Ϊ0.3 NA |
| B�������£�1 mol Cl2��������NaOH��Һ��Ӧ��ת�Ƶĵ�����ΪNA |
| C����ͬ��ͬѹ�£�3.2 g O2��0.4 g H2������ͬ����� |
| D��1 mol Na2O2�к��е�������������Ϊ4NA |




