��Ŀ����
Na2SO3�����ڿ����о������ױ��ʣ�
I��д��Na2SO3�����ڿ����б��ʵĻ�ѧ����ʽ
��ʵ������������ Na2SO3 ������Ʒ�Ƿ��Ѿ����ʣ���ķ���Ϊ
��ѡ������װ����ҩƷͨ���ⶨ����SO2���������ɼ���Na2SO3������Ʒ�Ĵ��ȣ�
��Ӧԭ����H2SO4��Ũ��+Na2SO3=Na2SO4+SO2��+H2O
��ѡ�õ�ҩƷ����Ũ���� ��Ũ���� �ۼ�ʯ�� �ܿ���
��ѡ�õ�װ�ã�ͬһװ�ÿ��ظ�ѡ�ã���
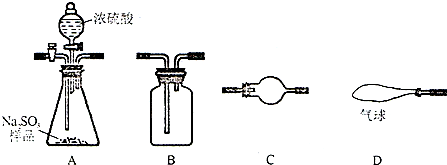
��1������ѡ����������˶������������������±�������Ӧ����������������Լ� ��������A װ���е�ҩƷ��������Ҫ��д���ɲ�������
��2����ȡa gNa2S03������Ʒ������ʵ����ɺ������SO2������Ϊ5g���Na2SO3������Ʒ����������Ϊ ��
I��д��Na2SO3�����ڿ����б��ʵĻ�ѧ����ʽ
��ʵ������������ Na2SO3 ������Ʒ�Ƿ��Ѿ����ʣ���ķ���Ϊ
��ѡ������װ����ҩƷͨ���ⶨ����SO2���������ɼ���Na2SO3������Ʒ�Ĵ��ȣ�
��Ӧԭ����H2SO4��Ũ��+Na2SO3=Na2SO4+SO2��+H2O
��ѡ�õ�ҩƷ����Ũ���� ��Ũ���� �ۼ�ʯ�� �ܿ���
��ѡ�õ�װ�ã�ͬһװ�ÿ��ظ�ѡ�ã���

��1������ѡ����������˶������������������±�������Ӧ����������������Լ� ��������A װ���е�ҩƷ��������Ҫ��д���ɲ�������
| ʵ��װ�ã�����ĸ�� | D | ||||||
| װ������ʢҩƷ������ţ� | �� |
���㣺����ʵ�鷽�������,�������ʵ����ʼ��ۺ�Ӧ��,�Ƶ���Ҫ������
ר�⣺ʵ�������
���������������ƹ��屻��������Ϊ�����ƣ�
��Na2SO3 ������Ʒ�Ѿ����ʣ�˵��������������ӣ�������ʹ�����ữ���Ȼ�����Һ������ɫ�����ķ�����֤�Ƿ��������������
��1������ʵ��ԭ�����ʵ�鲽�裬�ٸ��ݲ���������������ͨ���ⶨ����SO2���������ɼ���Na2SO3������Ʒ�Ĵ��ȣ����Զ�������Ҫȫ������ʯ�����գ������ֲ�����������еĶ�����̼��ˮ�����Լ���Ӧ������ˮ��������ͨ�����Ҫ�ȳ�������̼��ˮ����������D��A֮�仹Ӧ����һ����ʯ�ҵĸ���װ�ã�A���ɶ�������ʱ��Ҫͨ��Ũ����������ˮ���������Ҫ��һ����ʯ�ҵĸ���װ�÷�ֹ����Ŀ�������Ӱ��ⶨ�����
��2���������غ�����������Ƶ��������ٸ�����������=
��100%���м��㣮
��Na2SO3 ������Ʒ�Ѿ����ʣ�˵��������������ӣ�������ʹ�����ữ���Ȼ�����Һ������ɫ�����ķ�����֤�Ƿ��������������
��1������ʵ��ԭ�����ʵ�鲽�裬�ٸ��ݲ���������������ͨ���ⶨ����SO2���������ɼ���Na2SO3������Ʒ�Ĵ��ȣ����Զ�������Ҫȫ������ʯ�����գ������ֲ�����������еĶ�����̼��ˮ�����Լ���Ӧ������ˮ��������ͨ�����Ҫ�ȳ�������̼��ˮ����������D��A֮�仹Ӧ����һ����ʯ�ҵĸ���װ�ã�A���ɶ�������ʱ��Ҫͨ��Ũ����������ˮ���������Ҫ��һ����ʯ�ҵĸ���װ�÷�ֹ����Ŀ�������Ӱ��ⶨ�����
��2���������غ�����������Ƶ��������ٸ�����������=
| m(Na2SO3) |
| m(��) |
���
�⣺���������ƹ��屻��������Ϊ�����ƣ��ʷ�Ӧ����ʽΪ��2Na2SO3+O2=2Na2SO4���ʴ�Ϊ��2Na2SO3+O2=2Na2SO4��
��Na2SO3 ������Ʒ�Ѿ����ʣ�˵��������������ӣ�����ȡ������Ʒ�ܽ⣬���������������ữ���Ȼ�����Һ����ַ�Ӧ�����а�ɫ�������ڣ�֤��Na2SO3�Ѿ����ʣ����ް�ɫ������֤��Na2SO3δ���ʣ�
�ʴ�Ϊ��ȡ������Ʒ�ܽ⣬���������������ữ���Ȼ�����Һ����ַ�Ӧ�����а�ɫ�������ڣ�֤��Na2SO3�Ѿ����ʣ����ް�ɫ������֤��Na2SO3δ���ʣ�
��1��ͨ���ⶨ����SO2���������ɼ���Na2SO3������Ʒ�Ĵ��ȣ����Զ�������Ҫȫ������ʯ�����գ������ֲ�����������еĶ�����̼��ˮ�����Լ���Ӧ������ˮ��������ͨ�����Ҫ�ȳ�������̼��ˮ����������D��A֮�仹Ӧ����һ����ʯ�ҵĸ���װ�ã�A���ɶ�������ʱ��Ҫͨ��Ũ���������ղ�����ˮ���������Ҫ��һ����ʯ�ҵĸ���װ�÷�ֹ����Ŀ�������Ӱ��ⶨ������ʴ�Ϊ��
��2���������غ㣬���������Ƶ�����Ϊ
��126g/mol=
g������Na2SO3������Ʒ����������Ϊ
��100%=
��100%���ʴ�Ϊ��
��100%��
��Na2SO3 ������Ʒ�Ѿ����ʣ�˵��������������ӣ�����ȡ������Ʒ�ܽ⣬���������������ữ���Ȼ�����Һ����ַ�Ӧ�����а�ɫ�������ڣ�֤��Na2SO3�Ѿ����ʣ����ް�ɫ������֤��Na2SO3δ���ʣ�
�ʴ�Ϊ��ȡ������Ʒ�ܽ⣬���������������ữ���Ȼ�����Һ����ַ�Ӧ�����а�ɫ�������ڣ�֤��Na2SO3�Ѿ����ʣ����ް�ɫ������֤��Na2SO3δ���ʣ�
��1��ͨ���ⶨ����SO2���������ɼ���Na2SO3������Ʒ�Ĵ��ȣ����Զ�������Ҫȫ������ʯ�����գ������ֲ�����������еĶ�����̼��ˮ�����Լ���Ӧ������ˮ��������ͨ�����Ҫ�ȳ�������̼��ˮ����������D��A֮�仹Ӧ����һ����ʯ�ҵĸ���װ�ã�A���ɶ�������ʱ��Ҫͨ��Ũ���������ղ�����ˮ���������Ҫ��һ����ʯ�ҵĸ���װ�÷�ֹ����Ŀ�������Ӱ��ⶨ������ʴ�Ϊ��
| ʵ��װ�ã�����ĸ�� | C | A | B | C | C | ||
| װ������ʢҩƷ������ţ� | �� | �� | �� | �� |
| 5g |
| 64g/mol |
| 315 |
| 32 |
| ||
| a |
| 315 |
| 32a |
| 315 |
| 32a |
���������⿼�����ʵı������⣬�����������Ʊ������������Ƽ�����������Ӽ���ķ���Ϊ���Ĺؼ���ע������غ㷨���㼴�ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д� �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д�
�����Ŀ
�������ӷ���ʽ������ǣ�������
| A����Mg��HCO3��2��Һ�м�������� NaOH��Һ��Mg2++2HCO3-+4OH-=Mg��OH��2��+2CO32-+2H2O |
| B���������ᱵ�����м���ϡ���3BaSO3+2H++2NO3-=3BaSO4��+2NO��+H2O |
| C����������Һ�еμӹ���ϡ���[Ag��NH3��2]++2H+=Ag++2NH4+ |
| D����NH4HSO4ϡ��Һ����μ���Ba��OH��2ϡ��Һ��SO42-�պó�����ȫ��Ba2++2OH-+NH4++H++SO42-=BaSO4��+NH3?H2O+H2O |
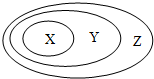 ����ͼ��ʾ��һЩ���ʻ�����Ĵ�����ϵ�в���ȷ���ǣ�������
����ͼ��ʾ��һЩ���ʻ�����Ĵ�����ϵ�в���ȷ���ǣ�������| X | Y | Z | |
| A | ���� | ������ | ������ |
| B | ����� | ���ӻ����� | ������ |
| C | �������� | ��ɢϵ | ����� |
| D | ���������� | ���������� | ������ |
| A��A | B��B | C��C | D��D |
ij��Һ�п��ܺ�������6�������еļ��֣�NH4+��A13+��Mg2+��CO32-��Cl-��SO42-��Ϊȷ����Һ����ɣ���ȡ100mL�ֳ����ȷ���Һ��������ʵ�飺
��1�����һ����Һ�м��� AgNO3��Һ�г���������
��2����ڶ�����Һ�м�������NaOH��Һ��ַ�Ӧ�����յõ�����0.58g��ͬʱ�ռ�������0.03mol��������ȫ������Һ���ݳ�����
��3�����������Һ�м�������BaCl2��Һ�������ữ����ַ�Ӧ�õ�����6.99g��
�ɴ˿�֪�����й���ԭ��Һ��ɵ���ȷ�����ǣ�������
��1�����һ����Һ�м��� AgNO3��Һ�г���������
��2����ڶ�����Һ�м�������NaOH��Һ��ַ�Ӧ�����յõ�����0.58g��ͬʱ�ռ�������0.03mol��������ȫ������Һ���ݳ�����
��3�����������Һ�м�������BaCl2��Һ�������ữ����ַ�Ӧ�õ�����6.99g��
�ɴ˿�֪�����й���ԭ��Һ��ɵ���ȷ�����ǣ�������
| A����Һ��SO42-��Ũ����0.3 mol/L |
| B����Һ��һ������A13+��NH4+ |
| C��һ��������Mg2+�����ܴ���A13+ |
| D��һ������Cl- ���ܺ�CO32- |
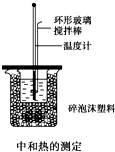 50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺