��Ŀ����
ʵ����Ҫ����100mL��2mol/L��KOH��Һ���ش�
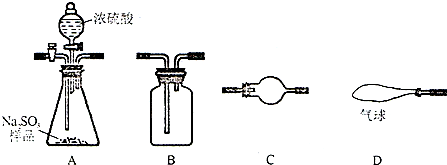
��1��һ���ò����������� ������ĸ��ʾ��
A���ձ� B��200mL����ƿ C����Ͳ D����ͷ�ι� E��������F��100mL����ƿ
��2������ʱӦ��ȡKOH���������Ϊ g��
��3��ʵ�鿪ʼʱ�����������ƿ ��
��4�����в�����˳���ǣ�����ĸ��ʾ�� ��
A ��ȴ B���� Cϴ�� D���� E�ܽ� Fҡ�� Gת��
��5������ƿ������ˮϴ����δ�����ֱ������������Һ����ʵ���� ����С��ޡ��� Ӱ�죮
��1��һ���ò�����������
A���ձ� B��200mL����ƿ C����Ͳ D����ͷ�ι� E��������F��100mL����ƿ
��2������ʱӦ��ȡKOH���������Ϊ
��3��ʵ�鿪ʼʱ�����������ƿ
��4�����в�����˳���ǣ�����ĸ��ʾ��
A ��ȴ B���� Cϴ�� D���� E�ܽ� Fҡ�� Gת��
��5������ƿ������ˮϴ����δ�����ֱ������������Һ����ʵ����
���㣺��Һ������
ר�⣺ʵ����
��������1������һ�����ʵ���Ũ����Һ���ƵIJ�������õ���ʵ��������
��2�������ݴ���cV=n��m=nM�����ȡKOH�����������
��3������ƿ���в�������ʹ��ǰҪ�����Ƿ�©ˮ��
��4��һ�����ʵ���Ũ����Һ���ƵIJ���Ϊ���㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�
��5������ƿ������ˮϴ����δ��������ʵ���������Һ�������Ӱ�죬��˲�Ӱ��Ũ�ȣ�
��2�������ݴ���cV=n��m=nM�����ȡKOH�����������
��3������ƿ���в�������ʹ��ǰҪ�����Ƿ�©ˮ��
��4��һ�����ʵ���Ũ����Һ���ƵIJ���Ϊ���㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�
��5������ƿ������ˮϴ����δ��������ʵ���������Һ�������Ӱ�죬��˲�Ӱ��Ũ�ȣ�
���
�⣺��1���ܽ���Ҫ�ձ�����Ͳ����������ת����Ҫ100ml����ƿ����������������Ҫ��ͷ�ιܣ����һ���ò�����������200ml����ƿ��
�ʴ�Ϊ��B��
��2��n��KOH��=2mol/L��0.1L=0.2mol��m��KOH��=0.2mol��56g/mol=11.2g��
�ʴ�Ϊ��11.2��
��3������ƿ���в�������ʹ��ǰҪ�����Ƿ�©ˮ���ʴ�Ϊ���Ƿ�©ˮ��
��4��һ�����ʵ���Ũ����Һ���ƵIJ���Ϊ���㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�
�ʴ�Ϊ��BEAGCDF��
��5������ƿ������ˮϴ����δ��������ʵ���������Һ�������Ӱ�죬��˲�Ӱ��Ũ�ȣ��ʴ�Ϊ���ޣ�
�ʴ�Ϊ��B��
��2��n��KOH��=2mol/L��0.1L=0.2mol��m��KOH��=0.2mol��56g/mol=11.2g��
�ʴ�Ϊ��11.2��
��3������ƿ���в�������ʹ��ǰҪ�����Ƿ�©ˮ���ʴ�Ϊ���Ƿ�©ˮ��
��4��һ�����ʵ���Ũ����Һ���ƵIJ���Ϊ���㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�
�ʴ�Ϊ��BEAGCDF��
��5������ƿ������ˮϴ����δ��������ʵ���������Һ�������Ӱ�죬��˲�Ӱ��Ũ�ȣ��ʴ�Ϊ���ޣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ��漰ʵ�鲽���ʵ���������������Ŀ��飬�ѶȲ���

��ϰ��ϵ�д�
 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A��������ˮ�ĵ����һ����������� |
| B��ǿ����ʵ�ˮ��Һ��������һ�����������ˮ��Һ�ĵ�������ǿ |
| C��ij���������ǵ���ʣ���һ���Ƿǵ���� |
| D������ˮ��ǿ����ʣ���ˮ��Һ��ȫ����������� |
���з�Ӧ�����ӷ���ʽ��д��ȷ���ǣ�������
| A����AgCl����Һ�еμ�������Һ����ɫ������ɺ�ɫ��2AgCl��s��+S2-��aq���TAg2S��s��+2Cl-��aq�� | ||||
| B����NaClO��Һ��ͨ������SO2���壺2ClO-+SO2+H20�T2HClO+SO32- | ||||
C��������ͭ�缫�������ͭ��Һ��Cu2++2H2O
| ||||
| D����������ˮ��S2-+2H2O?H2S+2OH- |
���ֽ��������30g��Ͷ��������ϡ�����У���Ӧ����������0.5mol���������е����ֽ���Ϊ��������
| A��þ���� | B�������� |
| C��þ���� | D��þ��п |
����������PHֵ�����ǣ�������
| A��0.0001mol/L��HCl |
| B��0.00001mol/L��H2SO4 |
| C��0.0000001mol/L��NaOH |
| D����ˮ |