��Ŀ����
�ο����Т١�����ش����⡣������ֵ��ָʹ
| ������ | �������� | ţ�� | ���� | Ӳ������ | ���� |
����ֵ | 190 | 180 | 192 | 226 | 193 | 193 |
��ֵ | 90 | 182 | 38 | 38 | 5 | 126 |
(1)������(C17H33COO)
(2)�����Т١��۵������������ʵ��Ĵʾ䡣
���������ͱȻ�����������__________�ࣻ
�ڻ��ͱ�ţ��������__________�ࣻ
��Ӳ�����͵ĵ�ֵС��ԭ����____________________��
(3)Ϊʹ��ֵΪ180��
(4)�����нṹʽ������������������ֵΪ430����nΪ���٣���������淴Ӧ����ʽ��
![]() +KOH
+KOH![]() ( )+( )
( )+( )
������(1)�ݷ�Ӧ��(C17H33COO)![]()
������ֵΪx,��
(2)��������ɵã���֬��Է�������ԽС������ֵԽ����֬��Է�������Խ������ֵԽС������ֵԽ�����ͼ���ĿԽ�ࣻ��ֵԽС�������ͼ���ĿԽС��
(3)
V=![]() ��
��
(4)�����ķ���ʽΪCn+3H2n+6O2����Է�������Ϊ
12 n+36+2n+32+6=14n+74,KOH����Է�������Ϊ56���ù�ϵʽ(14 n+74)��56=100��43,n=4��
��ӦʽΪC4H9COOC2H5+KOH![]() C4H9COOK+C2H5OH
C4H9COOK+C2H5OH
�𰸣�(1)190 (C17H33COO)![]() C3H5(OH)3+
C3H5(OH)3+
(2)�ٲ����ͼ� �ڵͼ�֬������ �۲����ͼ���
(3)
(4)n=

 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
| |||||||||||||||||||

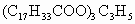 (������884)�γɵ��ͣ���������������ʱ������ֵΪ____________��д���䷴Ӧ����ʽ________________________��
(������884)�γɵ��ͣ���������������ʱ������ֵΪ____________��д���䷴Ӧ����ʽ________________________��

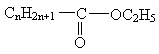 ��KOH��(����)��(����)��
��KOH��(����)��(����)��
