题目内容
9.工业上用CO2和H2反应合成二甲醚.已知:CO2(g)+3H2(g)?CH3OH(g)+H2O(g)△H1=-49.1kJ•mol-1
2CH3OH(g)?CH3OCH3 (g)+H2O(g)△H2=-24.5kJ•mol-1
写出CO2(g)和H2(g)转化为CH3OCH3(g)和H2O(g)的热化学方程式2CO2(g)+6H2(g)?CH3OCH3(g)+3H2O(g)△H=-122.7kJ•mol-.
分析 由①CO2(g)+3H2(g)?CH3OH(g)+H2O(g)△H1=-49.1kJ•mol-1
②2CH3OH(g)?CH3OCH3 (g)+H2O(g)△H2=-24.5kJ•mol-1
结合盖斯定律可知,①×2+②得到2CO2(g)+6H2(g)?CH3OCH3(g)+3H2O(g),以此来解答.
解答 解:由①CO2(g)+3H2(g)?CH3OH(g)+H2O(g)△H1=-49.1kJ•mol-1
②2CH3OH(g)?CH3OCH3 (g)+H2O(g)△H2=-24.5kJ•mol-1
结合盖斯定律可知,①×2+②得到2CO2(g)+6H2(g)?CH3OCH3(g)+3H2O(g),则△H=(-49.1kJ•mol-1)×2+(-24.5kJ•mol-1)=-122.7 kJ•mol-1,即热化学方程式为2CO2(g)+6H2(g)?CH3OCH3(g)+3H2O(g)△H=-122.7 kJ•mol-1,
故答案为:2CO2(g)+6H2(g)?CH3OCH3(g)+3H2O(g)△H=-122.7 kJ•mol-.
点评 本题考查热化学方程式的书写,为高频考点,把握物质的量与热量的关系、焓变为解答的关键,侧重分析与应用能力的考查,注意盖斯定律的应用,题目难度不大.

练习册系列答案
 课程达标测试卷闯关100分系列答案
课程达标测试卷闯关100分系列答案 新卷王期末冲刺100分系列答案
新卷王期末冲刺100分系列答案 全能闯关100分系列答案
全能闯关100分系列答案
相关题目
20.下列有关描述不正确的是( )
| A. | 钠在空气和氯气中燃烧,火焰皆呈黄色,但生成固体颜色不同 | |
| B. | 新制饱和氯水和浓硝酸光照下均会有气体产生,其成分中都有氧气 | |
| C. | 浓硫酸具有较强酸性,能使Cu转化为Cu2+ | |
| D. | 灼烧NaOH固体时不能使用瓷坩埚,因为坩埚中的SiO2能与NaOH反应 |
17.NA为阿伏伽德罗常数的值.下列说法正确的是( )
| A. | 2.4 g镁在空气中完全燃烧生成MgO和Mg3N2,转移的电子数为0.8 NA | |
| B. | 标准状况下,5.6 L二氧化碳气体中含有的氧原子数为0.5 NA | |
| C. | 8.7 g MnO2与40 mL 10 mol/L的浓盐酸充分反应,生成的氯气分子数为0.1 NA | |
| D. | 0.1 L 0.5 mol/L CH3COOH溶液中含有的氢离子数为0.05 NA |
14.某研究小组探究SO2和Fe(NO3)3溶液的反应.请回答:

(1)A中滴加浓硫酸之前应进行的操作是打开弹簧夹,通入一段时间N2,再关闭弹簧夹,目的是排尽装置内的空气.
(2)装置B中产生了白色沉淀,其成分是BaSO4.
已知SO2不与BaCl2溶液反应,该研究小组对产生白色沉淀的原因进行了假设:
假设1:SO2与Fe3+反应;假设2:在酸性条件下SO2与NO3-反应;
假设3:Fe3+、NO3-同时氧化SO2.
【设计方案、验证假设】
(3)某同学设计实验验证假设2,请帮他完成下表中内容.
经验证假设2成立,则验证过程中发生的离子方程式是4SO2+NO3-+5H2O+4Ba2+=4BaSO4↓+NH4++6H+.
(提示:NO3-在不同条件下的还原产物较复杂,此条件未见气体产生)
【思考与交流】
若假设1、假设2都成立,你是否同意假设3,并简述理由否,未检验Fe3+与此条件下NO3-的氧化性强弱或未检验是否生成Fe2+.

(1)A中滴加浓硫酸之前应进行的操作是打开弹簧夹,通入一段时间N2,再关闭弹簧夹,目的是排尽装置内的空气.
(2)装置B中产生了白色沉淀,其成分是BaSO4.
已知SO2不与BaCl2溶液反应,该研究小组对产生白色沉淀的原因进行了假设:
假设1:SO2与Fe3+反应;假设2:在酸性条件下SO2与NO3-反应;
假设3:Fe3+、NO3-同时氧化SO2.
【设计方案、验证假设】
(3)某同学设计实验验证假设2,请帮他完成下表中内容.
| 实验步骤 | 预期的现象和结论 |
| ①测定B中实验所用混合溶液的 ②配制具有相同pH的稀硝酸与 BaCl2的混合液并通入适当的N2 ③将SO2通入上述溶液中 | 若出现白色沉淀则假设2成立 若不出现白色沉淀则假设2不成立 |
(提示:NO3-在不同条件下的还原产物较复杂,此条件未见气体产生)
【思考与交流】
若假设1、假设2都成立,你是否同意假设3,并简述理由否,未检验Fe3+与此条件下NO3-的氧化性强弱或未检验是否生成Fe2+.
1.某品牌茶叶中铁元素的检验可经过以下四个步骤完成:将茶叶灼烧灰化→用浓硝酸溶解茶叶灰→过滤得到的滤液→检验滤液中的Fe3+.图是该实验可能用到的实验用品.有关该实验的说法中错误的是( )


| A. | 第一步需选用仪器①、②和⑨,仪器①的名称叫坩埚 | |
| B. | 第二步用浓硝酸溶解茶叶灰,可能将Fe2+氧化成Fe3+ | |
| C. | 要完成第三步,需选用④、⑤和⑦,除夹持仪器外还缺滤纸 | |
| D. | 第四步,用试剂⑧检验滤液中的Fe3+,溶液中生成血红色沉淀 |
18.同温同压下,同物质的量的CH4气体与CO体积比是( )
| A. | 3:1 | B. | 1:1 | C. | 1:3 | D. | 2:3 |
19.下列叙述正确的是( )
| A. | 强电解质都易溶于水,所以BaSO4是弱电解质 | |
| B. | 氨气的水溶液可以导电,但氨气属于非电解质 | |
| C. | 使用催化剂可以让不自发的化学反应自发进行 | |
| D. | 电解质溶液的导电过程属于物理变化 |
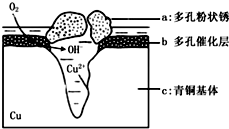 图为青铜器在潮湿环境中发生电化学腐蚀的原理示意图.
图为青铜器在潮湿环境中发生电化学腐蚀的原理示意图.