��Ŀ����
7��������һ����Ҫ�Ļ���ԭ�ϣ��㷺����ҩ���������߷��Ӻϳɵȹ�ҵ��ijѧϰС���ͬѧ���Ը�����Ϊԭ����ˮ��-����-ˮ��ѭ��������ȡ���ᣮ�������ϵ�֪����1���������Ҵ���ˮ���������ѣ��ӷ����������ڱ������Ȼ�̼��
��2��������к�ǿ�Ļ�ԭ��

�����������Ϣ�ش��������⣺
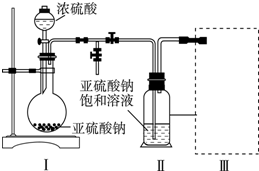
��1������-ˮ�����������ͼ1��װ���н��еģ�ָ��װ��A������������ƿ��
��2������-ˮ������У���������������Ӧ��ʱ�����������ͬ������£��ı䷴Ӧ�¶��Կ��췴Ӧ�¶ȶԲ�����ʵ�Ӱ�죬�������ͼ2��ʾ����ѡ����ѵķ�Ӧ�¶�Ϊ70�棬ʵ����������μӹ�����¶ȹ��ߣ������²�������½�����ԭ���Dz����ױ�Ũ���������������
��3���õ��ֲ�Ʒ��ϴ��ʱѡ������ ��ϴ�Ӽ����������ӷ���������
��4��Ϊ��ȷ����Ʒ��H2C2O4•2H2O��M=126g/mol���Ĵ��ȣ���ȡ10.0g������Ʒ�����250mL��Һ��ÿ��ʵ��ʱȷ��ȡ25.00mL������Һ������������ϡ���ᣬ��0.1mol/LKMnO4����Һ�ζ���ƽ�еζ����Σ��ظ��ζ����Σ�ÿ������KMnO4����Һ��������±���ʾ
| ��һ�� | �ڶ��� | ������ | |
| �����mL�� | 16.10mL | 16.60mL | 15.90mL |
���ڲ��ᴿ�Ȳⶨ��ʵ������У�����˵����ȷ���ǣ�CD��
A�����Ʊ�Һʱ����KMnO4���������ʲ������Һ��Ӧ������ʹʵ�����ƫ��
B����ϴ�ζ���ʱ��Ӧ�ӵζ����Ͽڼ������������Һ��ʹ�ζ����ڱڳ����ϴ
C���ζ�ʱ���۾�ע����ƿ���۲���Һ��ɫ�仯
D���ζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬��ʹʵ�����ƫ��
���жϵζ��Ѿ��ﵽ�յ�ķ����ǣ�����ƿ�е������һ�θ�����ر�Һ����ƿ����Һ���dz��ɫ�Ұ���Ӻ�ɫ��
�ܲ��ᾧ��Ĵ���Ϊ50.4%��
���� ��1���������ṹ��������֪����AΪ������ƿ��
��2�����ݲ���Ļ��������ʱ�ж���ѷ�Ӧ�¶ȣ�Ũ���ᡢ�������ǿ�����ԣ������������
��3�������������Ҵ���ˮ���������ѣ��������ӷ���������ϴ�ӣ�
��4�������������¸�����ؽ���������Ϊ������̼����������ԭΪ�����ӣ�
��A�����Ʊ�Һʱ����KMnO4���������ʲ������Һ��Ӧ�������ĸ��������Һ���ƫ��
B����ϴ�ζ���ʱ������Ҫ�������������Һ������ҩƷ���˷ѣ�
C���ζ�ʱ���۾�ע����ƿ���۲���Һ��ɫ�仯���жϷ�Ӧ�յ㣻
D���ζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬��ʹ���ĸ��������Һ���������ƫ��
���������Ը��������Һ���Ϻ�ɫ�����ᷴӦ��ϣ��������һ�θ�����ر�Һ����ƿ����Һ���dz�죬��Ӧ�����յ㣻
�ܵ�2�������������������ϴ�Ӧ��������������ƽ��ֵΪ�������Ը��������Һ���������n=cV���㷴Ӧ�����ĸ�����ص����ʵ��������ݷ���ʽ5H2C2O4+2MnO4-+6H+=2Mn2++10CO2��+8H2O����25mL��������ʵ������ټ���ԭ��Ʒ�в�������ʵ���������������ᾧ��Ĵ��ȣ�
��� �⣺��1���������ṹ��������֪����AΪ������ƿ��
�ʴ�Ϊ��������ƿ��
��2������ͼ3��֪�����¶�Ϊ70��ʱ�������������ߣ�����ѡ�����ѷ�Ӧ�¶���70�棬
Ũ���ᡢ�������ǿ�����ԣ������������ᣬ����μӹ�����¶ȹ��ߣ������²�������½���
�ʴ�Ϊ��70�棻�����ױ�Ũ���������������
��3�������������Ҵ���ˮ���������ѣ��������ӷ���������ϴ�ӣ�
�ʴ�Ϊ�����ѣ��ӷ���������
��4�������������¸�����ؽ���������Ϊ������̼����������ԭΪ�����ӣ���Ӧ���ӷ���ʽΪ��5H2C2O4+2MnO4-+6H+=2Mn2++10CO2��+8H2O��
�ʴ�Ϊ��5H2C2O4+2MnO4-+6H+=2Mn2++10CO2��+8H2O��
���ݲ�����Һ������Լ���ȡ��Һ�ľ�ȷ��ѡ����Ӧ��������
��A�����Ʊ�Һʱ����KMnO4���������ʲ������Һ��Ӧ�������ĸ��������Һ���ƫ��ʹʵ�����ƫƫ�ߣ���A����
B����ϴ�ζ���ʱ������Ҫ�������������Һ������ҩƷ���˷ѣ���B����
C���ζ�ʱ���۾�ע����ƿ���۲���Һ��ɫ�仯���жϷ�Ӧ�յ㣬��C��ȷ��
D���ζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬��ʹ���ĸ��������Һ���������ƫ��ʹʵ�����ƫ�ߣ���D��ȷ��
��ѡ��CD��
���������Ը��������Һ���Ϻ�ɫ�����Եζ��յ���ж������ǣ�����ƿ�е������һ�θ�����ر�Һ����ƿ����Һ���dz��ɫ�Ұ���Ӻ�ɫ��
�ʴ�Ϊ������ƿ�е������һ�θ�����ر�Һ����ƿ����Һ���dz��ɫ�Ұ���Ӻ�ɫ��
�ܵ�2�������������������ϴ�Ӧ�������������Ը��������Һ���Ϊ$\frac{��16.10+15.90��ml}{2}$=16.00mL����Ӧ�����ĸ�����ص����ʵ�����0.016L��0.1mol/L=0.0016mol�����ݷ���ʽ��5H2C2O4+2MnO4-+6H+=2Mn2++10CO2��+8H2O����֪25mL��Һ�в�������ʵ�����0.0016mol��$\frac{5}{2}$=0.004mol�����ԭ��Ʒ�в�������ʵ�����0.004mol��$\frac{250ml}{25ml}$=0.04mol��������=0.04mol��126g/mol=5.04g�����Բ���Ĵ�����$\frac{5.04g}{10.0g}$��100%=50.4%��
�ʴ�Ϊ��50.4%��
���� ���⿼���л����Ʊ�ʵ�鷽����ƣ��漰��ѧ��������װ�õķ������ۡ�ʵ��������ѡ����ơ����ʺ����IJⶨ��������ԭ��Ӧ�ζ��ȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ�������Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ���ʷ�����ѧ��Ӧ�������������ı仯�����������仯�����ʱ仯���ǻ�ѧ�仯 | |
| B�� | 101kPaʱ��2H2��g��+O2��g���T2H2O��l����H=-571.6kJ•mol-1��H2��g����ȼ����Ϊ285.8kJ•mol-1 | |
| C�� | ����ͬ�����µ�ϡ��Һ�У�1molHCl��1molHNO3�ֱ�������NaOH��ַ�Ӧ���ų���������� | |
| D�� | H2+Cl2�T2HCl�������仯���������ͼ��ʾ |
| A�� | �� | B�� | �٢ڢ� | C�� | �٢ڢܢ� | D�� | �٢ڢ� |

�����õ����й������б����£�
| ��� | �۵�/�� | �е�/�� | �ܶȣ�20�棩/g•cm-3 | �ܽ��� |
| �� | 5.5 | 80 | 0.88 | ����ˮ |
| ������ | 5.7 | 210.9 | 1.205 | ������ˮ |
| Ũ���� | - | 83 | 1.4 | ������ˮ |
| Ũ���� | - | 338 | 1.84 | ������ˮ |
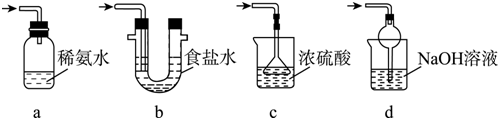
��1�����ƻ���Ӧ���ձ����ȼ���Ũ���ʵ��װ���г���������������������ܣ������������ܣ����棨���������ƣ�����ѹ��Һ©�����ŵ���ʹ�������˳�����£�
��2����Ӧ�¶ȿ�����50��60���ԭ���Ƿ�ֹ����Ӧ��������Ӧ������������²㣨��ϡ����ߡ��¡���������ڷ������Ͳ�Ʒ�IJ��������Ƿ�Һ��
��3����Na2CO3��Һϴ��֮����������ˮϴ��ʱ��������֤Һ����ϴ����ȡ���һ��ϴ��Һ������Һ�м����Ȼ��ƣ��������ɣ�˵����ϴ����
��4������D������Ϊ��ˮ�Ȼ��ƻ���ˮ����þ��
��5�������ۡ�ϡ����������������Ph-NO2��ʾ ����Ӧ������Ⱦ���м��屽����Ph-NH2�����䷴Ӧ�Ļ�ѧ����ʽΪC6H5NO2+3Fe+6HCl��C6H5NH2+3FeCl2+2H2O��
| A�� | ��pH=1����Һ�У�SO32-��Cl-��NO3-��Na+ | |
| B�� | ����ʹ��̪������Һ�У�Na+��Cl-��NO3-��K+ | |
| C�� | ��1 mol•L-1��NaAlO2��Һ�У�K+��Ba2+��SO42-��OH- | |
| D�� | ��1 mol•L-1��AlCl3��Һ�У�NH4+��Ag+��Na+��NO3- |
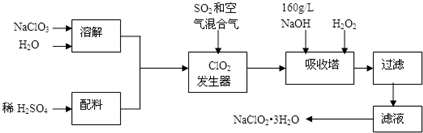
 A��ʯ���ѽ���Ҫ����֮һ������������ں���һ��ʯ�ͻ�����չˮƽ�ı�־��
A��ʯ���ѽ���Ҫ����֮һ������������ں���һ��ʯ�ͻ�����չˮƽ�ı�־��