��Ŀ����
16����֪AΪ����ɫ���壬T��RΪ���ֳ�������;�ܹ�Ľ������ʣ�D�Ǿ��д��Եĺ�ɫ���壬C����ɫ��ζ�����壬H�ǰ�ɫ��������ش��������⣮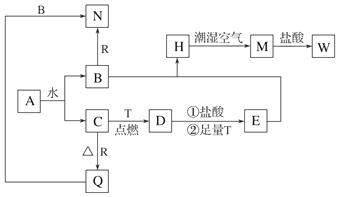
��1��д���������ʵĻ�ѧʽ��DFe3O4��QAl2O3
��2��A��ˮ��Ӧ�Ļ�ѧ����ʽ2Na2O2+2H2O=4NaOH+O2����
��3����Ҫȷ��E��Һ���Ƿ���W���ʣ���ѡ�Լ�Ϊb��
a����ˮ��KSCN��Һ b��KSCN��Һ c��Ũ��ˮ d�����Ը��������Һ
��4��B��E��͵õ�H���ڳ�ʪ�����б��M�Ĺ����У��ɹ۲쵽���������ɰ�ɫ����������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��
��5��B��R��Ӧ����N�����ӷ���ʽ2Al+2OH-+2H2O=2AlO2-+3H2��W��Һ�뵥��T��Ӧ�����ӷ���ʽFe+2Fe3+=3Fe2+��
���� AΪ����ɫ���壬��A�ܺ�ˮ��Ӧ����A��Na2O2��A��ˮ��Ӧ����NaOH��O2��C����ɫ��ζ�����壬��C��O2��B��NaOH��D�Ǿ��д��Եĺ�ɫ���壬��D��Fe3O4��Fe��������ȼ��������������������T��Fe��R��������Ӧ����������Q��Q�ܺ�NaOH��Һ��Ӧ����Q��Al2O3��R��Al��N��NaAlO2��H�ǰ�ɫ���������ڳ�ʪ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ����M����H��Fe��OH��2��M��Fe��OH��3��Fe3O4��HCl��Fe��Ӧ��õ�E��EΪFeCl2��Fe��OH��3��HCl��Ӧ����W����W��FeCl3���Դ˽����⣮
��� �⣺��1�������Ϸ�����֪DΪFe3O4��QΪAl2O3���ʴ�Ϊ��Fe3O4��Al2O3��
��2��A��Na2O2��A��ˮ��Ӧ����NaOH��O2������ʽΪ2Na2O2+2H2O=4NaOH+O2�����ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
��3��EΪFeCl2��W��FeCl3��ȷ��E��Һ���Ƿ���W���ʣ��ɼ���KSCN���飬�ʴ�Ϊ��b��
��4��Fe��OH��2���ȶ����ױ��������������������ɹ۲쵽������Ϊ���ɰ�ɫ����������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��
�ʴ�Ϊ�����ɰ�ɫ����������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��
��5������NaOH��Һ��Ӧ����ƫ�����ƺ����������ӷ�Ӧ����ʽΪ2Al+2OH-+2H2O�T2AlO2-+3H2����W��FeCl3��T��Fe�����߷�Ӧ�����ӷ���ʽΪFe+2Fe3+=3Fe2+��
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����Fe+2Fe3+=3Fe2+��
���� ������Na��Al��Fe���仯����Ϊ���忼���˽���Ԫ�ؼ��仯������ƶϣ�����H����ɫ�仯��A����ɫ�����ʡ�D������Ϊͻ�ƿڲ��������ϵķ��������ƶϣ���Ϥ���������ǽⱾ��ؼ����ٽ�����ʼ��ת���������Ŀ�Ѷ��е�

 ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д�| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ�I1=738kJ/mol I2=1451kJ/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص����������������������4 |
| F ��ǰ������ԭ�ӵ����Ų�ͼ�е�����������Ԫ�� |
| G�����ڱ��ĵ�ʮһ�� |
 ��
����2��C��������������Ԫ��ԭ�ӵĵ�һ�������ɴ�С��˳����Mg��Al��Na����Ԫ�ط�����գ���
��3��B��D�ĵ縺����Դ�С��B����D���á����ڡ�����С�ڡ����ڡ���գ���
��4����������Ԫ���У�λ��s������1�֣�λ��p������4�֣�
��5��DE3�ĵ���ʽΪ
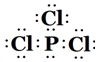 ��
�� | A�� | 16gO2ռ�е����ԼΪ11.2L | |
| B�� | 22.4LH2���а����ӵ������������ | |
| C�� | �ڱ�״���£�44.8LH2O������ԼΪ36g | |
| D�� | 11gCO2���״����5.6LHCl������ͬ�ķ����� |
| ʱ��/s | 0 | 20 | 40 | 60 | 80 | 100 |
| c��NO2��/mol•L-1 | 0.00 | 0.12 | 0.20 | 0.26 | 0.30 | 0.30 |
| A�� | 20��40s�ڣ�v��N2O4��=0.004mol/��L•s�� | |
| B�� | ����ͬ�����£���ʼʱ���������г������0.80 molNO2���ﵽƽ���NO2��ת����Ϊ75% | |
| C�� | ��Ӧ��ƽ��ʱ�����յ�����Ϊ15.9 kJ | |
| D�� | 100 sʱ��ͨ��0.40 mol N2O4������ƽ��ʱN2O4��ת�������� |
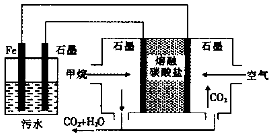
| A�� | Fe�缫�ĵ缫��ӦʽΪ��Fe-2e-�TFe2+ | |
| B�� | ͨ�������ʯī�缫�ĵ缫��ӦʽΪO2+2CO2+4e-�T2CO32- | |
| C�� | ͨ������ʯī�缫�ĵ缫��ӦʽΪ��CH4+100H+-8e-�TCO32-+7H2O | |
| D�� | Ϊ��ǿ��ˮ�ĵ���������������ˮ�м���������ҵ��ʳ�� |
| A�� |  ������ͺ�ˮ ������ͺ�ˮ | B�� | 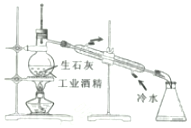 �ù�ҵ�ƾ���ȡ��ˮ�ƾ� �ù�ҵ�ƾ���ȡ��ˮ�ƾ� | ||
| C�� | 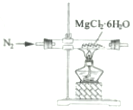 ��ȡMgCl2���� ��ȡMgCl2���� | D�� | 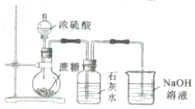 ���������Ũ���ᷴӦ������CO2 ���������Ũ���ᷴӦ������CO2 |
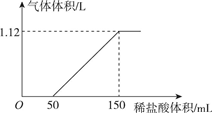 ��ij̼���ƺ�̼�����ƵĻ����Һ����μ���0.05mol/L��ϡ���ᣬ����ϡ����������״���²�����������Ĺ�ϵ��ͼ��ʾ��
��ij̼���ƺ�̼�����ƵĻ����Һ����μ���0.05mol/L��ϡ���ᣬ����ϡ����������״���²�����������Ĺ�ϵ��ͼ��ʾ��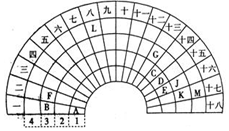 Ԫ�����ڱ�����ʽ���ֶ�������ͼ������Ԫ�����ڱ���һ���֣�ǰ�����ڵ�Ԫ�أ����Ա���ѧ����Ԫ�����ڱ����ش��������⣺
Ԫ�����ڱ�����ʽ���ֶ�������ͼ������Ԫ�����ڱ���һ���֣�ǰ�����ڵ�Ԫ�أ����Ա���ѧ����Ԫ�����ڱ����ش��������⣺ ��
�� ��
��