��Ŀ����
1����һ��ɫ����Һ����ȷ���Ƿ����������ӣ�K+��Mg2+��Al3+��Fe2+��Ba2+��NO3-��SO42-��Cl-��I-��HCO3-��ȡ����Һ��������ʵ�飬����˵������ȷ���ǣ�������
| ʵ�鲽�� | ʵ������ |
| ��ȡ��������Һ���Ӽ���ʯ����Һ | ��Һ���ɫ |
| ��ȡ��������Һ������ͭƬ��Ũ���ᣬ���� | ����ɫ������������������Ա�ɺ���ɫ |
| ��ȡ��������Һ������BaCl2��Һ | �а�ɫ�������� |
| ��ȡ���е��ϲ���Һ������AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����ϡ���� |
| ��ȡ��������Һ������NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ�����������ܽ� |
| A�� | ��Һ��һ�����ڵ�������SO42-��Mg2+��Al3+��Cl- | |
| B�� | ��Һ��һ�����ڵ�������NO3-��SO42-��Mg2+��Al3+ | |
| C�� | ��Һ�п϶������ڵ�������Fe2+��HCO3-��I-��SO42- | |
| D�� | ��Һ�п϶������ڵ�������Fe2+��HCO3-��Mg2+��I-��Ba2+ |
���� ������ɫ����Һ������Fe2+���ɣ�1��֪��Һ�����ԣ�����HCO3-���ɣ�2��֪��NO���ɣ�ԭ��Һ�к�NO3-����һ������Fe2+��I-�����л�ԭ�ԣ����ɣ�3��֪��SO42-���ڣ���ԭ��Һ����Ba2+���ɣ�4������ȷ���Ƿ�Cl-����3������Cl-���ɢ�֪��Mg2+��Al3+��
��� �⣺������ɫ����Һ������Fe2+��
���ݣ�1��ȡ��������Һ���Ӽ�����ɫʯ����Һ����Һ�Ժ�ɫ��˵����Һ��ʾ���ԣ�����HCO3-�����ڣ�
���ݣ�2��ȡ��������Һ����Ũ������CuƬ��Ũ���ᣬ��������ɫ������������壨NO�����������Ա�ɺ���ɫ��������������˵����Һ�к���NO3-����һ��������I-��
���ݣ�3��ȡ��������Һ����BaCl2��Һ���а�ɫ�������ɣ�����SO42-��
���ݣ�4��ȡ��3���е��ϲ���ҹ����AgNO3�����ȶ��İ�ɫ�������ɣ��Ҳ�����ϡ���ᣬ��3������Һ�к���Cl-�����ڸ��ݣ�3���м������Ȼ���������Cl-������ȷ��ԭ��Һ���Ƿ����Cl-��
���ݣ�5��ȡ��������Һ������NaOH��Һ�а�ɫ�������ɣ���NaOH����ʱ�����������ܽ⣬��ԭ��Һ�к���Mg2+��Al3+��
���Ͽ�֪����Һ��һ�����ڵ������ǣ�NO3-��SO42- Mg2+��Al3+����Һ�п϶������ڵ������ǣ�I-��Ba2+��Fe2+��HCO3-������ȷ�����У�K+��Cl-��
��ѡB��
���� ���⿼�鳣�����ӵļ���ͼ����Ǹ߿��еij������ͣ��ۺ���ǿ������������ѧ����������������ʵ���������������Ԫ�ػ����������ǹؼ���ע�����ճ������ӵļ��飬�Ѷ��еȣ�

| A�� | N2�ĵ���ʽ��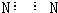 | B�� | S2-�Ľṹʾ��ͼ�� | ||
| C�� | ������Ľṹʽ��H-O-Cl | D�� | ${\;}_{40}^{65}$Zr��${\;}_{40}^{67}$Zr��ͬ�ֺ��� |
�ټӳɷ�Ӧ ��ˮ�ⷴӦ ��������Ӧ ���кͷ�Ӧ ��������Ӧ��
| A�� | ֻ�Т٢� | B�� | ֻ�Тڢۢ� | C�� | ֻ�Т٢ۢܢ� | D�� | ȫ�� |
 ��ʹ������к͵ζ����ⶨ���۰״���������g/100mL����
��ʹ������к͵ζ����ⶨ���۰״���������g/100mL������ʵ�鲽�裺
��1����ȡ10.00mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100mL����ƿ�����������ƣ��ж��ݣ�ҡ�ȼ��ô���״���Һ��
��2������ʽ�ζ���ȡ����״���Һ20.00mL����ƿ�У������еμ�2�η�̪��ָʾ����
��3����ȡʢװ0.100 0mol/L NaOH��Һ�ļ�ʽ�ζ��ܵij�ʼ���������Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ0.70 mL��
�ζ�������Һ����ɫǡ�ñ�Ϊ��ɫ�����ڰ�����ڲ���ɫʱ��ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Σ�
��ʵ���¼������ ml��
| ����� | 1 | 2 | 3 | 4 |
| V����Ʒ�� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH�� | 15.95 | 15.00 | 15.05 | 14.95 |
��1����ͬѧ�ڴ�������ʱ����ã�ƽ�����ĵ�NaOH��Һ����� V=��15.95+15.00+15.05+14.95��/4mL=
15.24mL��ָ�����ļ���IJ�����֮������1�εζ�������Դ����쳣ֵ��Ӧ��ȥ������ȷ���ݴ������ɵ����۰״�������=4.50����g/100mL����д��������̣�
��2���ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ�����ab����д��ţ���
a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
c����ƿ�м������״���Һ���ټ�����ˮ d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦����
| A�� | C2H6��C4H10һ����ͬϵ�� | |
| B�� | C2H4��C4H8һ����ͬϵ�� | |
| C�� | C3H6��ֻ��ʾһ������ | |
| D�� | ϩ���и�ͬϵ����̼������������ͬ |
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | ��ʯī�缫���Mg��NO3��2��Pb�� NO3��2 �Ļ��Һ | �Ƚ�ȷ��Ǧ��þ�Ľ������ǿ�� |
| B | ����ͬ�¶��±���Na2CO3��Һ�ͱ���Na2SiO3��Һ��pH | ȷ��̼����Ԫ�طǽ�����ǿ�� |
| C | �����£���pH�ƲⶨŨ��Ϊ0.1mol•L-1NaClO��Һ��0.1mol•L-1CH3COONa��Һ��pH | �Ƚ�HClO��CH3COOH������ǿ�� |
| D | �����£��ֱ���2֧�Թ��м�����ͬ�������ͬŨ�ȵ�Na2S2O3��Һ���ٷֱ������ͬ�����ͬŨ�ȵ�ϡ���� | �о�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��ɫ�� | B�� | ������ | C�� | Ӫ��ǿ���� | D�� | ��ζ�� |
| A�� | ��CH3CH2Br��NaOH��Һ���ȣ���ȴ��ȡ�ϲ���Һ��AgNO3��Һ���۲��Ƿ��������ɫ����������CH3CH2Br��NaOH��Һ�Ƿ�����Ӧ | |
| B�� | ��ʵ���ң����Ҵ���Ũ����Ļ���ﹲ�ȷ�����Ӧ��������ʹ����KMnO4��Һ��ɫ�����壬������һ������ϩ | |
| C�� | ���������������ӵĻ��Һ�м��������ռ���Һ�������ú��Һ���ɳ�ȥ���еı��� | |
| D�� | ����������Һ��������ȩ���Թ��г�ֻ�Ϻ��Թܷ�����ˮԡ�м��ȣ����Թ��ڱ����й������������� |
 һ���¶��������Ϊ5L���ܱ������з������淴Ӧ��
һ���¶��������Ϊ5L���ܱ������з������淴Ӧ��