��Ŀ����
12����1��0.02mol/L�Ĵ�����Һ��0.01mol/L������������Һ�������ϣ���Ϻ���Һ��pH=6������Һ�г�ˮ����������Ũ�ȣ���CH3COOH���ɴ�С��˳��Ϊc��CH3COO-����c��Na+����c��CH3COOH����c��H+����c��OH-������CH3COO-��-c��Na+��=9.9��10-7mol/L��д����ȷ����������2��ijѧ����0.2000mol•L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ���ⶨ���ƫ�ߣ���ԭ�������ABCD��
A�����Ʊ���Һ�Ĺ���NaOH�л���KOH����
B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�����������ȷ
C��ʢװδ֪Һ����ƿ������ˮϴ��������δ֪Һ��ϴ
D���ζ����յ����ʱ�����ֵζ��ܼ��촦����һ����Һ
��3��ij�¶���ˮ�����ӻ�ΪKW=1��10-13���������¶���pH=11��NaOH��Һa L��pH=1��ϡ����b L��ϣ����Ϻ���Һ�����С�仯���Բ��ƣ��������û��Һ��pH=2����a��b=9��2��
���� ��1����Ӧ������Ϊ��Ũ�ȵĴ����ƺʹ��ᣬ���Һ��pH=6����Һ�����ԣ�������Ũ��Ϊc��H+��=1��10-6mol/L��������������Ũ��Ϊ��c��OH-��=1��10-8mol/L����ϵ���غ���м��㣻
��2������c�����⣩=$\frac{V��������c������}{V�����⣩}$��������������V����������Ӱ�죬�Դ��ж�Ũ�ȵ���
��3�������û��Һ��pH=2������c��H+��=$\frac{c���ᣩV���ᣩ-c���V���}{V���ᣩ+V���}$������������ȣ�
��� �⣺��1����Ӧ������Ϊ��Ũ�ȵĴ����ƺʹ��ᣬ���Һ��pH=6����Һ�����ԣ���c��H+����c��OH-�����ɵ���غ�c��CH3COO-��+c��OH-��=c��Na+��+c��H+����֪��c��CH3COO-����c��Na+��������Һ������Ũ�ȹ�ϵΪ��c��CH3COO-����c��Na+����c��CH3COOH����c��H+����c��OH-������֪������Ũ��Ϊc��H+��=1��10-6mol/L��������������Ũ��Ϊ��c��OH-��=1��10-8mol/L�����ݵ���غ��֪��c��CH3COO-��-c��Na+��=c��OH-��-c��H+��=1��10-6mol/L-1��10-8mol/L=9.9��10-7mol/L��
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��CH3COOH����c��H+����c��OH-����9.9��10-7mol/L��
��2��A�����Ʊ���Һ�Ĺ���NaOH�л���KOH���ʣ����ڵζ�ʱ���ĵ�����ƫ�٣�������������һ��ʱ�����ĵı���Һ�����ƫ����ⶨ���ƫ��A��ȷ��
B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ������Һ�����ƫ����ⶨ���ƫ��B��ȷ��
C��ʢװδ֪Һ����ƿ������ˮϴ��������δ֪Һ��ϴ����ƿ����������ʵ���ƫ�ζ�ʱ���ĵı���Һ�����ƫ�����Բⶨ���ƫ��C��ȷ��
D���ζ����յ����ʱ�����ֵζ��ܼ��촦����һ����Һ�������ĵı���Һ�����ƫ�����Բⶨ���ƫ��D��ȷ��
�ʴ�Ϊ��ABCD��
��3��ij�¶���ˮ�����ӻ�ΪKW=1��10-13�������û��Һ��pH=2������$\frac{0.1b-0.01a}{a+b}$=0.01�����a��b=9��2��
�ʴ�Ϊ��9��2��
���� ���⿼������ϵĶ����жϡ�pH���йؼ��㡢����к͵ζ��������ȣ��������Һ������Լ�����غ��ж�����Ũ�ȴ�С��ע�⣨1��3����ˮ�����ӻ�������10-13������10-14������ᵼ�´���Ϊ�״��㣻�����ڿ���ѧ���Ի���֪ʶ��Ӧ�������ͼ���������

| A�� | ������Һɱ����Ӿ���е����� | |
| B�� | ��CaCl2�ڻ�·��Ļ�ѩ | |
| C�� | ��ʯ�����������ʴ������ | |
| D�� | �ô���������β���е�CO��NOת��Ϊ������ |
| A�� | ������0.4 mol/L HB��Һ�� 0.2 mol/L NaOH��Һ�������Ϻ���Һ��pH=3��������Һ������Ũ�ȵĴ�С˳��Ϊ��c��B-����c��H+����c��Na+����c��OH-�� | |
| B�� | ��Ũ�ȵ�����ϡ��Һ������������ �������� �۴��� ��̼������ �������� �ޱ����ƣ����ǵ�pH��С��������Ϊ���ۢݢ٢ܢڢ� | |
| C�� | ������0.1 mol/L��������Һ ��NH4Al��SO4��2 ��NH4Cl ��NH3•H2O ��CH3COONH4��c ��NH4+���ɴ�С��˳���ǣ��ڣ��٣��ܣ��� | |
| D�� | ��25��ʱ����a mol•L-1�İ�ˮ��0.01 mol•L-1������������Ϸ�Ӧʱ��Һ��c��NH4+��=c��Cl-�����ú�a�Ĵ���ʽ��ʾNH3•H2O�ĵ��볣��Kb=$\frac{1{0}^{-9}}{a-0.01}$ |
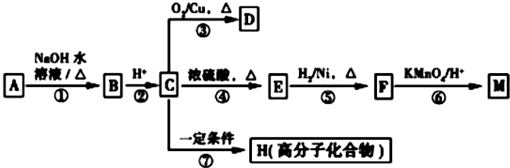
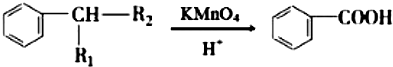 ��R1��R2��ʾ��������ԭ�ӣ�
��R1��R2��ʾ��������ԭ�ӣ�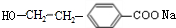 ��
��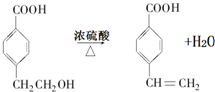 ��
��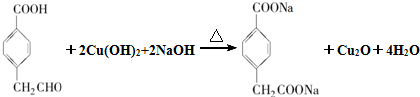 ��
��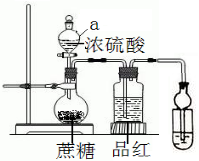 ����ƿ��20�����ǣ����μ�������ˮ��20mLŨ���ᣬ������ڣ�������ͣ��γ����ɶ�ĺ��������ƿ���̣�Ʒ����Һ��ɫ�䵭����ش�
����ƿ��20�����ǣ����μ�������ˮ��20mLŨ���ᣬ������ڣ�������ͣ��γ����ɶ�ĺ��������ƿ���̣�Ʒ����Һ��ɫ�䵭����ش�
 ��
��