��Ŀ����
14��X��Y��Z��M��R��W��Ϊ���ڱ���ǰ�����ڵ�Ԫ�أ�X�Ļ�̬ԭ����Χ�����Ų�ʽΪ3s2��Yԭ�ӵ�L���Ӳ��P�ܼ�����һ���չ����ZԪ�صĻ�̬ԭ���������3��δ�ɶԵ��ӣ��������2�����ӣ� M ��ԭ�ӵ�2p�������1�����ӵ������������������ӵ����������෴�� WΪ����Ԫ�أ����̬ԭ����Χ�����Ų��ɶԵ�������δ�ɶԵ�����4�����ش��������⣨��ػش����Ԫ�ط��ű�ʾ������1��X�Ļ�̬ԭ����12���˶�״̬��ͬ�ĵ��ӣ�
��2��ZM3-�����ӿռ乹��Ϊƽ�������Σ�����Z���ӻ���ʽΪSP2�ӻ���
��3��W��Ԫ�ط���MnW��YM���γ������W��YM��4����W��YM��4��W�Ļ��ϼ�Ϊ0����YM���ӻ�Ϊ�ȵ���������ӵĻ�ѧʽΪCN-��C22-��O22+��
��4��W���ʵľ����ڲ�ͬ�¶���������ԭ�Ӷѻ���ʽ�������ֱ���ͼA��B��ʾ��

��ͼB��ԭ�Ӷѻ���ʽΪ�����������ܶѻ���A��B��Wԭ�ӵ���λ��֮��Ϊ2��3��
��A��B�������ⳤ�ֱ�Ϊa cm��b cm����A��B���־�����ܶ�֮��Ϊb3��2a3��
��ͼA�Ķѻ���ʽ�ռ�������$\frac{\sqrt{3}��}{8}��100%$���ú��е�ʽ�ӱ�ʾ��
���� X��Y��Z��M��W��Ϊ���ڱ���ǰ�����ڵ�Ԫ�أ�X�Ļ�̬ԭ����Χ�����Ų�ʽΪ3s2����XΪ�������ڢ�A��Ԫ�أ�ΪMgԪ�أ�
Y��̬ԭ�ӵ�L���Ӳ��p�ܼ�����һ���չ����ԭ�Ӻ�������Ų�Ϊ1s22s22p2����YΪCԪ�أ�
ZԪ�صĻ�̬ԭ���������3��δ�ɶԵ��ӣ��������2�����ӣ���Z���ڵڶ����ڣ������Ų�ʽΪ��1s22s22p3��ΪNԪ�أ�
M�Ļ�̬ԭ�ӵ�2p�������1�����ӵ������������������ӵ����������෴��ԭ�Ӻ�������Ų�Ϊ1s22s22p4����MΪOԪ�أ�
WΪ����Ԫ�أ��������̬ԭ����Χ�����Ų��ɶԵ�������δ�ɶԵ�����4������������Ų�Ϊ1s22s22p63s23p63d54s2����WΪMnԪ�أ��ݴ˽��н��
��� �⣺X��Y��Z��M��W��Ϊ���ڱ���ǰ�����ڵ�Ԫ�أ�X�Ļ�̬ԭ����Χ�����Ų�ʽΪ3s2����XΪ�������ڢ�A��Ԫ�أ�ΪMgԪ�أ�
Y��̬ԭ�ӵ�L���Ӳ��p�ܼ�����һ���չ����ԭ�Ӻ�������Ų�Ϊ1s22s22p2����YΪCԪ�أ�
ZԪ�صĻ�̬ԭ���������3��δ�ɶԵ��ӣ��������2�����ӣ���Z���ڵڶ����ڣ������Ų�ʽΪ��1s22s22p3��ΪNԪ�أ�
M�Ļ�̬ԭ�ӵ�2p�������1�����ӵ������������������ӵ����������෴��ԭ�Ӻ�������Ų�Ϊ1s22s22p4����MΪOԪ�أ�
WΪ����Ԫ�أ��������̬ԭ����Χ�����Ų��ɶԵ�������δ�ɶԵ�����4������������Ų�Ϊ1s22s22p63s23p63d54s2����WΪMnԪ�أ�
��1��XΪMgԪ�أ����̬ԭ�Ӻ�������Ų�Ϊ1s22s22p63s2��ÿһ�����ӵ��˶�״̬����ͬ���ʺ�����12���˶�״̬��ͬ�ĵ��ӣ��ʴ�Ϊ��12��
��2��ZM3-ΪNO3-��NO3-��Nԭ�ӵļ۲���Ӷ���Ϊ��$\frac{5+1}{2}$=3��û�йµ��Ӷԣ�����NO3-�Ŀռ乹��Ϊƽ�������Σ�Nԭ�Ӳ���sp2���ʣ�
�ʴ�Ϊ��ƽ�������Σ�sp2��
��3��W��YM���γ������W��YM��4ΪMn��CO��4��Mn��CO���γ������Fe��CO��4���ϼ۵Ĵ�����Ϊ0��CO�Ļ��ϼ۵Ĵ�����Ϊ0����Mn�Ļ��ϼ�Ϊ0��
���ݵȵ�����Ķ��壬CO�ĵȵ����������˫ԭ�ӷ��ӻ����ӣ��ҵ���������ȣ�������CO���ӻ�Ϊ�ȵ���������ӵĻ�ѧʽΪCN-��C22-��O22+��
�ʴ�Ϊ��Mn��0��CN-��C22-��O22+��
��4�����Զ���Mnԭ���о�����֮�����ԭ�Ӵ������ģ�ÿ������Ϊ12���湲�ã��������������ܶѻ���Mnԭ����λ��Ϊ12�����������ѻ���Mnԭ����λ��Ϊ8����A��B���ֶѻ�����λ��֮��Ϊ8��12=2��3��
�ʴ�Ϊ�������������ܶѻ���2��3��
��A��ԭ��ռ�����ĺͶ��㣬Ϊ���������ṹ��ԭ����Ϊ1+8��$\frac{1}{8}$=2��B��ռ�ݶ�������ģ�ԭ����Ϊ6��$\frac{1}{2}$+8��$\frac{1}{8}$=4����������������������������������ʵ�ʺ��е�Feԭ�Ӹ���֮��1��2��A��B�������ⳤ�ֱ�Ϊa cm��b cm������ֱ�Ϊa3cm3��b3cm3���������ܶȵ��ھ�������ԭ�ӵ�����������ıȣ�Ϊ $\frac{2m}{{a}^{3}}$��$\frac{4m}{{b}^{3}}$=b3��2a3��
�ʴ�Ϊ��b3��2a3��
��ͼA��ԭ����ĿΪ1+8��$\frac{1}{8}$=2��ԭ�Ӱ뾶Ϊr��ԭ�������Ϊ2��$\frac{4��{r}^{3}}{3}$���������Ϊ$��\frac{4r}{\sqrt{3}}��^{3}$���ռ�������=$\frac{ԭ�������}{�������}$��100%=$\frac{\sqrt{3}��}{8}��100%$��
�ʴ�Ϊ��$\frac{\sqrt{3}��}{8}��100%$��
���� ���⿼��λ�á��ṹ�����ʹ�ϵ���ۺ�Ӧ�á������ļ����֪ʶ����Ŀ�Ѷ��еȣ��ƶϸ�Ԫ��Ϊ���ؼ���ע������ԭ�ӽṹ��Ԫ�������ɡ�Ԫ�������ɵĹ�ϵ����4��Ϊ�ѵ㣬��Ҫ��ȷ��̯���ھ��������е�Ӧ�ã�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ����ǿ����HIO4��HBrO4��HClO4 | B�� | ԭ�Ӱ뾶��С��Al��P��N | ||
| C�� | ����ǿ����KOH��NaOH��LiOH | D�� | ������ǿ����Na��Mg��Al |
| A�� | $\frac{12n}{m}$ | B�� | $\frac{60n}{m}$ | C�� | $\frac{11m}{120n}$ | D�� | $\frac{120n}{11m}$ |
 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2•6C2H5OH
���й��л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/�� | 34.7 | 78.5 | 118 | 77.1 |
��1��Ũ��������ã������ᡢ��������ˮ��������ͬλ��18Oʾ�ٷ�ȷ����Ӧ����ˮ��������ԭ�ӵ��ṩ�ߣ�д���ܱ�ʾ18Oλ�õĻ�ѧ����ʽ��CH3COOH+CH3CH218OH
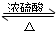 CH3CO18OC2H5+H2O��
CH3CO18OC2H5+H2O����2�����θ����C�������Ƿ�ֹ����������������Ӧǰ��D�м��뼸�η�̪����Һ�ʺ�ɫ�������������ԭ���ǣ������ӷ���ʽ��ʾ��CO32-+H2O?HCO3-+OH-��Ӧ������D�е���������Һ�ֲ㣬�ϲ���ɫ����Һ�壬�²���Һ��ɫ��dz��
��3����D�з���������������г�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ�������Ҵ����ټ��루�˿մ�����ѡ����ѡ��C��Ȼ����������ռ�77�����ҵ���֣��Եõ��ϴ���������������
A������������ B����ʯ�� C����ˮ������ D����ʯ�ң�
| A�� | 18gD2O�к��е���������Ϊ10NA | |
| B�� | 2L0.5mol/L������Һ�к�����������ĿΪNA | |
| C�� | ��״���£�22.4LSO3�����еķ�����ĿΪNA | |
| D�� | ����������ˮ��Ӧʱ����0.1mol����ת�Ƶĵ�����Ϊ0.2NA |

 �������������е�����������������Ϊ���Ӽ������ۼ���
�������������е�����������������Ϊ���Ӽ������ۼ��� ��ͼ��ʾ�����Թܷ���ʢ��25�汥��ʯ��ˮ���ձ��У��Թܿ�ʼ���뼸С��þƬ�����õιܵ���5mL�������Թ��У��Իش��������⣺
��ͼ��ʾ�����Թܷ���ʢ��25�汥��ʯ��ˮ���ձ��У��Թܿ�ʼ���뼸С��þƬ�����õιܵ���5mL�������Թ��У��Իش��������⣺
