��Ŀ����
4����0.1000mol/L������ⶨijNaOH��Һ�����ʵ���Ũ�ȣ��Իش���1���ü�ʽ�ζ�����ȡ20.00mL����Һ������������ƿ�У��ٵ����̪��
��2����0.1000mol/L������ζ�NaOH��Һ���ζ�ʱ����Ӧע����ƿ����Һ��ɫ�仯ֱ���ζ��յ㣻
��3�����²������ܵ������ⶨNaOH��ҺŨ��ƫ�͵��ǣ�ע�⣺��ѡ����ѡ���۷֣�BC��
A����ʽ�ζ���δ�ñ���Һ��ϴ
B��ҡ����ƿʱ����Һ�ηɽ�����
C����ȡ��������ʱ���ζ�ǰƽ�Ӷ������ζ����Ӷ���
��4��ijͬѧ�ζ�ʱ��¼���������£�
| ʵ���� | ����NaOH��Һ�����/mL | �ζ����ʱ��������������/mL |
| 1 | 20.00 | 22.40 |
| 2 | 20.00 | 22.42 |
| 3 | 20.00 | 22.38 |
���� ��1������Һ�ü�ʽ�ζ�����ȡ��
��2���ζ�ʱ�۾�Ӧע����ƿ����Һ��ɫ�仯�����ж��ζ��յ㣻
��3�����������������V�������ı仯������c�����⣩=$\frac{c��������V������}{V�����⣩}$������
��4���ȸ������ݵ���Ч�ԣ�Ȼ�����ƽ������V�����ᣩ�����Ÿ���c�����⣩=$\frac{c��������V������}{V�����⣩}$�����㣮
��� �⣺��1������ҺΪNaOH��Һ���Լ��ԣ��ü�ʽ�ζ�����ȡ20.00mL����Һ��
�ʴ�Ϊ����ʽ�ζ��ܣ�
��2���ζ�ʱ����Ӧע����ƿ����Һ��ɫ�仯��
�ʴ�Ϊ����ƿ����Һ��ɫ�仯��
��3��A����ʽ�ζ���δ�ñ���Һ��ϴ����Һ���ڱڵ�ˮϡ�ͣ�����c��������С�����V������������c�����⣩=$\frac{c��������V������}{V�����⣩}$������c������ƫ�ߣ���A����
B��ҡ����ƿʱ����Һ�ηɽ�����������Һ�����ʵ���ƫС�����V��������С������c�����⣩=$\frac{c��������V������}{V�����⣩}$������c������ƫ�ͣ���B��ȷ��
C����ȡ��������ʱ���ζ�ǰƽ�Ӷ������ζ����Ӷ��������V��������С������c�����⣩=$\frac{c��������V������}{V�����⣩}$������c������ƫ�ͣ���C��ȷ��
��ѡ��BC��
��4�����εζ��������������ֱ�Ϊ��22.40mL��22.42mL��22.38mL�����ݾ���Ч����ƽ������V��NaOH��=22.40mL��c�����⣩=$\frac{c��������V������}{V�����⣩}$=$\frac{0.1000mol/L��22.40mL}{20.00mL}$=0.1100mol•L-1��
�ʴ�Ϊ��0.112��
���� ���⿼���˵ζ���ʹ�á��к͵ζ��е���������ע��ݾ�c�����⣩=$\frac{c��������V������}{V�����⣩}$��������Ŀ�Ѷ��еȣ�

��ʵ��ǰ������Ũ��Ϊ0.1000mol•L-1����KMnO4����Һ�ζ�δ֪Ũ�ȵIJ��ᣮ
��1��д���ζ������з�����Ӧ�����ӷ���ʽΪ2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��
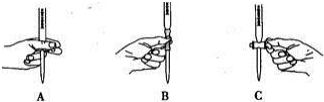
��2���ζ������в����ζ��ܵ�ͼʾ��ȷ����A��
��3������������KMnO4����Һʱ����������ƿ�Ŀ̶��ߣ���ʹ��õIJ�����ҺŨ��ƫ�ͣ��ƫ�ߡ�����ƫ�͡������䡱����
��ͨ���ζ�ʵ��õ�������Һ��Ũ��Ϊ0.2000mol•L-1���øò�����Һ���±����к���ʵ�飨ÿ��ʵ�������Һ��������Ϊ8mL����
| ʵ���� | �¶ȣ��棩 | ����������g�� | ���Ը��������Һ | ʵ��Ŀ�� a��ʵ��1��2�ǡ��� b��ʵ��1 ��3 ̽����Ӧ��Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ�죻 c��ʵ��1 ��4̽�������Ը÷�Ӧ���ʵ�Ӱ�죮 | |
| �����mL�� | Ũ�� ��mol•L-1�� | ||||
| 1 | 25 | 0.5 | 4 | 0.1000 | |
| 2 | 50 | 0.5 | 4 | 0.1000 | |
| 3 | 25 | 0.5 | 4 | 0.0100 | |
| 4 | 25 | 0 | 4 | 0.1000 | |
��5����С��ͬѧ��ʵ��1��3�ֱ����������ʵ�飬�������ʵ�����ݣ��ӻ�����ȿ�ʼ��ʱ����
| ʵ���� | ��Һ��ɫ����ʱ�䣨min�� | ||
| ��1 �� | ��2 �� | ��3 �� | |
| 1 | 14.0 | 13.0 | 11.0 |
| 3 | 6.5 | 6.7 | 6.8 |
��6����ʵ����ʹ�õĴ���Ӧѡ��MnSO4����MnCl2��ԭ��Ϊ2MnO4-+10Cl-+16H+=5Cl2��+2Mn2++8H2O�����ӷ���ʽ��ʾ����
| ʵ���� | ʵ���¶�/�� | c��Na2S2O3��/mol•L-1 | c��H2SO4��/mol•L-1 |
| �� | 25 | 0.1 | 0.1 |
| �� | 25 | 0.2 | 0.1 |
| �� | 25 | 0.1 | 0.2 |
| �� | 50 | 0.2 | 0.1 |
| �� | 50 | 0.1 | 0.1 |
̽��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬Ӧѡ��٢ڻ�٢ۻ�ܢݣ���ʵ���ţ���
��ͬʱѡ��٢ڢ���Һ����ǵ�ʱ�䣬̽���Ƚϸı䲻ͬ��Ӧ��Ũ�ȶԷ�Ӧ���ʵ�Ӱ��Ի�ѧ��Ӧ���ʵ�Ӱ�죮
| A�� | δ֪Ũ����Һ�õζ�����ȡ���ζ���������ˮϴ�Ӻ�û�ô�����Һ��ϴ | |
| B�� | װ����Һ����ƿ������ˮϴ�Ӻ�û�ô���Һ��ϴ | |
| C�� | ��ȡ��Һ�����ʱ����ʼ���Ӱ�Һ�棬ȡҺ����ʱ���Ӱ�Һ�� | |
| D�� | �ζ���ʼ�ζ��ܼ�������ݣ������յ�ʱ��������� |
| A�� | ��AԪ�صĵ縺�Դ��ϵ�����С����AԪ�صĵ�һ�����ܴ��ϵ�����С | |
| B�� | �縺�ԵĴ�С������Ϊ����Ԫ�صĽ����Ժͷǽ�����ǿ���ij߶� | |
| C�� | NaH�Ĵ�����֧�ֿɽ���Ԫ�ط��ڢ�A�Ĺ۵� | |
| D�� | ��ԭ����ֻ��һ�����ӣ�����ԭ��ֻ��һ��ԭ�ӹ�� |
 CO2��+2H2O��+4Cu����������ȫ��Ӧ��Ӳ�ʲ����ܵ�����������4.8g������Ӧ������ͨ��2L0.lmol/L�ij���Ca(OH��2��Һ��������գ����ɳ���10g����
CO2��+2H2O��+4Cu����������ȫ��Ӧ��Ӳ�ʲ����ܵ�����������4.8g������Ӧ������ͨ��2L0.lmol/L�ij���Ca(OH��2��Һ��������գ����ɳ���10g����