��Ŀ����
9������KMnO4��Һ������ᣨH2C2O4����Һ��Ӧ��ij̽��С�����÷�Ӧ��������Һ��ɫ��ʧ�����ķ������о�Ӱ�췴Ӧ���ʵ����أ���ʵ��ǰ������Ũ��Ϊ0.1000mol•L-1����KMnO4����Һ�ζ�δ֪Ũ�ȵIJ��ᣮ
��1��д���ζ������з�����Ӧ�����ӷ���ʽΪ2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��
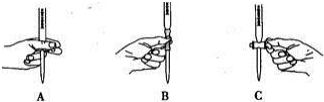
��2���ζ������в����ζ��ܵ�ͼʾ��ȷ����A��
��3������������KMnO4����Һʱ����������ƿ�Ŀ̶��ߣ���ʹ��õIJ�����ҺŨ��ƫ�ͣ��ƫ�ߡ�����ƫ�͡������䡱����
��ͨ���ζ�ʵ��õ�������Һ��Ũ��Ϊ0.2000mol•L-1���øò�����Һ���±����к���ʵ�飨ÿ��ʵ�������Һ��������Ϊ8mL����
| ʵ���� | �¶ȣ��棩 | ����������g�� | ���Ը��������Һ | ʵ��Ŀ�� a��ʵ��1��2�ǡ��� b��ʵ��1 ��3 ̽����Ӧ��Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ�죻 c��ʵ��1 ��4̽�������Ը÷�Ӧ���ʵ�Ӱ�죮 | |
| �����mL�� | Ũ�� ��mol•L-1�� | ||||
| 1 | 25 | 0.5 | 4 | 0.1000 | |
| 2 | 50 | 0.5 | 4 | 0.1000 | |
| 3 | 25 | 0.5 | 4 | 0.0100 | |
| 4 | 25 | 0 | 4 | 0.1000 | |
��5����С��ͬѧ��ʵ��1��3�ֱ����������ʵ�飬�������ʵ�����ݣ��ӻ�����ȿ�ʼ��ʱ����
| ʵ���� | ��Һ��ɫ����ʱ�䣨min�� | ||
| ��1 �� | ��2 �� | ��3 �� | |
| 1 | 14.0 | 13.0 | 11.0 |
| 3 | 6.5 | 6.7 | 6.8 |
��6����ʵ����ʹ�õĴ���Ӧѡ��MnSO4����MnCl2��ԭ��Ϊ2MnO4-+10Cl-+16H+=5Cl2��+2Mn2++8H2O�����ӷ���ʽ��ʾ����
���� ��1��������ؾ���ǿ�����ԣ��ܰѲ��������ɶ�����̼����������ԭ�ɶ��������ӣ�
��2�����ݵζ��ܵ�ʹ�ù����жϣ�
��3����������KMnO4����Һʱ����������ƿ�Ŀ̶��ߣ���Һ�����ƫС�����Ƶ���Һ��Ũ��ƫ�ζ�ʱ���ĵĸ��������Һ�����ƫС��
��4��ʵ��1��2ֻ���¶Ȳ�ͬ������������ȫ��ͬ��
��5��������ص����ʵ�����ͬ��Ũ�Ȳ�ͬ�IJ�����Һ������̽����Ӧ��Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ�죻
��6�����������£�������������ܽ�������������������
��� �⣺��1��������ؾ���ǿ�����ԣ��Ѳ����е�C��+3��������+4�۵Ķ�����̼��MnԪ�ش�+7�۱仯��+2�۵������ӣ����ڲ����������2��Cԭ�ӣ����ݵ�ʧ�����غ㣬������������ķ�Ӧ����Ϊ 5��2���ʷ�Ӧ�ķ���ʽΪ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��
�ʴ�Ϊ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��
��2�����ݵζ��ܵ�ʹ�ù��ζ�ʱ�����ְ�ס�ζ��ܵĻ�������Һ�εĵγ�����ֹ���������������ͼA��ʾ������
�ʴ�Ϊ��A��
��3����������KMnO4����Һʱ����������ƿ�Ŀ̶��ߣ���Һ�����ƫС�����Ƶ���Һ��Ũ��ƫ�ζ�ʱ���ĵĸ��������Һ�����ƫС�������ĵĸ�����ص����ʵ���ƫС����2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��֪���ⶨ�IJ�������ʵ���ƫС������õIJ�����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��4��ʵ��1��2��Ӧ��������ȫ��ͬ��ֻ���¶Ȳ�ͬ��Ŀ�ľ�����̽���¶Ȳ�ͬ�Է�Ӧ���ʵ�Ӱ�죬
�ʴ�Ϊ��̽���¶Ȳ�ͬ�Է�Ӧ���ʵ�Ӱ�죻
��5������ݱ����е���ɫʱ�䳤�����ж�Ũ�ȴ�С�뷴Ӧ���ʵĹ�ϵ�������������ص����ʵ�����ͬ��Ũ�Ȳ�ͬ�IJ�����Һ��
�ʴ�Ϊ������������ͬʱ�����õ����������ĸ��������������ͬŨ�ȵ�����������Һ��Ӧ��������Һ��ɫʱ�䣻
��6�����������£�������������ܽ���������������������������ԭ�ɶ��������ӣ���Ӧ����ʽΪ2MnO4-+10Cl-+16H+=5Cl2��+2Mn2++8H2O��
�ʴ�Ϊ��2MnO4-+10Cl-+16H+=5Cl2��+2Mn2++8H2O��
���� ���⿼����̽���¶ȡ�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�졢Ũ����ʱ��仯�����ߣ���Ŀ�Ѷ��еȣ�������ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�������������

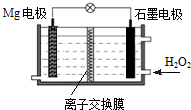 Mg-H2O2��ؿ��������˼�ʻ��DZ�������õ���Ժ�ˮΪ�������Һ��ʾ��ͼ��ͼ������˵����ȷ���ǣ�������
Mg-H2O2��ؿ��������˼�ʻ��DZ�������õ���Ժ�ˮΪ�������Һ��ʾ��ͼ��ͼ������˵����ȷ���ǣ�������| A�� | Mg�缫�Ǹõ�ص����� | B�� | H2O2��ʯī�缫�Ϸ���������Ӧ | ||
| C�� | �õ�ص��ܷ�ӦΪ��Mg+H2O2�TMg��OH��2 | D�� | ��Һ��Cl-�������ƶ� |
��1���ü�ʽ�ζ�����ȡ20.00mL����Һ������������ƿ�У��ٵ����̪��
��2����0.1000mol/L������ζ�NaOH��Һ���ζ�ʱ����Ӧע����ƿ����Һ��ɫ�仯ֱ���ζ��յ㣻
��3�����²������ܵ������ⶨNaOH��ҺŨ��ƫ�͵��ǣ�ע�⣺��ѡ����ѡ���۷֣�BC��
A����ʽ�ζ���δ�ñ���Һ��ϴ
B��ҡ����ƿʱ����Һ�ηɽ�����
C����ȡ��������ʱ���ζ�ǰƽ�Ӷ������ζ����Ӷ���
��4��ijͬѧ�ζ�ʱ��¼���������£�
| ʵ���� | ����NaOH��Һ�����/mL | �ζ����ʱ��������������/mL |
| 1 | 20.00 | 22.40 |
| 2 | 20.00 | 22.42 |
| 3 | 20.00 | 22.38 |
| A�� | �ζ�ǰ�ζ����������ݣ��յ����ʱ�����ݣ��������ƫС | |
| B�� | ������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫ�� | |
| C�� | ��ʪ���pH��ֽ����Һ��pH���ⶨֵһ������� | |
| D�� | �ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��������������У������к��ȵ�ֵƫС |
| A�� | ϡ���� | B�� | Ʒ����Һ | C�� | ���Ը������ | D�� | ��ˮ |