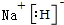��Ŀ����
9�� A��B��D��E��G��M����Ԫ��λ��Ԫ�����ڱ�ǰ�����ڣ�ԭ�����������������У�Ԫ��A��һ�ֺ��������ӣ�B�ĵ��ʼ��з��Ӿ�������ԭ�Ӿ��壬������DE2Ϊ����ɫ���壬G��ǰ�������е縺����С��Ԫ�أ�M��ԭ�Ӻ����������G��10��
A��B��D��E��G��M����Ԫ��λ��Ԫ�����ڱ�ǰ�����ڣ�ԭ�����������������У�Ԫ��A��һ�ֺ��������ӣ�B�ĵ��ʼ��з��Ӿ�������ԭ�Ӿ��壬������DE2Ϊ����ɫ���壬G��ǰ�������е縺����С��Ԫ�أ�M��ԭ�Ӻ����������G��10����ش��������⣺
��1����̬Gԭ�ӵĺ�������Ų�ʽ��1s22s22p63s23p64s1��M��Ԫ�����ڱ��е�λ���ǵ������ڵڢ�B�壬Ԫ��B��D��E�ĵ�һ�������ɴ�С��˳��ΪN��O��C����Ԫ�ط��ű�ʾ����
��2��Ԫ��A��E��ɵ������ӿռ乹��Ϊ�����Σ�������ABD�ĽṹʽΪH-C��N������Bԭ�ӵ��ӻ���ʽΪsp��
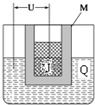
��3��G��M�ľ��徧���ṹ��ͼ��ʾ�������־�����ԭ�ӵ���λ��֮��Ϊ2��3��������M�ܶ�Ϊ8.9 g•cm-3����M�ľ����߳���$\root{3}{\frac{4��64}{8.9��6.02��1{0}^{23}}}$cm�����ؼ�����������
���� A��B��D��E��G��M����Ԫ��λ��Ԫ�����ڱ�ǰ�����ڣ�ԭ������������������Ԫ��A��һ�ֺ��������ӣ���AΪHԪ�أ�B�ĵ��ʼ��з��Ӿ�������ԭ�Ӿ��壬��BΪ̼Ԫ�أ�������DE2Ϊ����ɫ���壬��DΪNԪ�ء�EΪOԪ�أ�G��ǰ�������е縺����С��Ԫ�أ���GΪKԪ�أ�M��ԭ�Ӻ����������G��10����MΪCu��
��� �⣺A��B��D��E��G��M����Ԫ��λ��Ԫ�����ڱ�ǰ�����ڣ�ԭ������������������Ԫ��A��һ�ֺ��������ӣ���AΪHԪ�أ�B�ĵ��ʼ��з��Ӿ�������ԭ�Ӿ��壬��BΪ̼Ԫ�أ�������DE2Ϊ����ɫ���壬��DΪNԪ�ء�EΪOԪ�أ�G��ǰ�������е縺����С��Ԫ�أ���GΪKԪ�أ�M��ԭ�Ӻ����������G��10����MΪCu��
��1����̬Kԭ�ӵĺ�������Ų�ʽ��1s22s22p63s23p64s1��MΪCu����Ԫ�����ڱ��е�λ���ǵ������ڵڢ�B�壬ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���NԪ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�صģ��ʵ�һ�����ܣ�N��O��C��
�ʴ�Ϊ��1s22s22p63s23p64s1���������ڵڢ�B�壻N��O��C��
��2��Ԫ��A��E��ɵ�������ΪH3O+��Oԭ�Ӻ��йµ��Ӷ�Ϊ1���۲���Ӷ���Ϊ3+1=4����Ϊ�����νṹ��������HCN�ĽṹʽΪH-C��N������Cԭ���ӻ������ĿΪ2���ӻ���ʽΪsp�ӻ���
�ʴ�Ϊ�������Σ�H-C��N��sp�ӻ���
��3��G������ԭ����λ��Ϊ8��M�������Զ���ԭ���о�����֮�����ԭ���������ģ���λ��Ϊ$\frac{3��8}{2}$=12�������־�����ԭ�ӵ���λ��֮��Ϊ8��12=2��3��
Cu�ľ�����ԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4����������Ϊ4��$\frac{64}{6.02��1{0}^{23}}$g���������ܶ�Ϊ8.9 g•cm-3�������Ϊ4��$\frac{64}{6.02��1{0}^{23}}$g��8.9 g•cm-3=$\frac{4��64}{8.9��6.02��1{0}^{23}}$cm3����M�ľ����߳���$\root{3}{\frac{4��64}{8.9��6.02��1{0}^{23}}}$cm��
�ʴ�Ϊ��2��3��$\root{3}{\frac{4��64}{8.9��6.02��1{0}^{23}}}$��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų��������ܡ��ռ乹�����ӻ���ʽ�жϡ������ṹ�����ȣ�ע�����þ�̯�����о����йؼ��㣬��������ͬ����Ԫ�ص�һ�������쳣�����

| ���� | ��Է������� | �ܶ�/��g•mL-1�� | �е�/�� | ˮ���ܽ��� |
| CHCl3 | 119.5 | 1.50 | 61.3 | ���� |
| CCl4 | 154 | 1.59 | 76.7 | ���� |
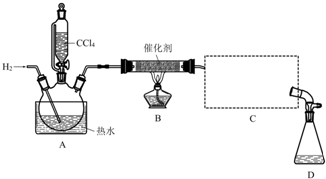
�ټ���װ�������ԣ��ڿ�ʼͨ��H2�� �۵�ȼB���ƾ��ƣ�
����A��ˮ���м�����ˮ����ͨC������װ�õ���ˮ��
��������ƿ�е���20mLCCl4��
��Ӧ������ֹͣ���ȣ���D����ƿ���ռ�����Һ��ֱ�������NaHCO3��Һ��ˮϴ�ӣ��ֳ��IJ������������ˮCaCl2���壬���ú���ˣ�
�߶���Һ�����������õ��ȷ�15g����ش�
��1��������ںͲ���۵�˳��ߵ�����ʵ���в����IJ����������Ϊ����ʱ����������������ը�����ɵ��ȷ±�����������
��2��B���з�����Ҫ��Ӧ�Ļ�ѧ����ʽΪCCl4+H2$��_{��}^{����}$CHCl3+HCl��
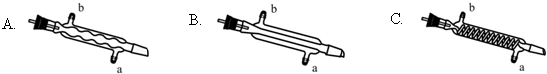
��3��C����Ӧѡ�õ�������ΪB����ѡ����ĸ������ˮӦ�Ӹ������ܵ�a���a����b�����ڽ��룮
��4��������У���ˮϴ�ӵ�Ŀ��Ϊϴ��NaHCO3��NaCl��
��5����ʵ���У��ȷµIJ���Ϊ61%��
��6���ȷ��ڿ������ܱ�������������HCl������COCl2�����÷�Ӧ�Ļ�ѧ����ʽΪ2CHCl3+O2=2COCl2+2HCl��
| A�� | ��-���屽ֻ��һ�� | |
| B�� | ������������ԭ����ͬһƽ���� | |
| C�� | ���ױ�û��ͬ���칹�� | |
| D�� | �����ܷ����ӳɷ�ӦҲ�ܷ���ȡ����Ӧ |
| A�� | ���ܶ�֮�ȵ��ڶ�Ӧ�����ʵ���֮�� | |
| B�� | ������֮�ȵ�����Է�������֮�� | |
| C�� | ���������������壬������ȵ�����Է��������ĵ���֮�� | |
| D�� | ��������������壬�����ʵ����ȵ�����Է�������֮�� |
| A�� | ���³�ѹ�£�32g O2��O3�Ļ����������ԭ����Ϊ2NA | |
| B�� | 100 mL 1 mol•L-1K2SO4��Һ�к��еļ�������Ϊ0.1NA | |
| C�� | 10����������ԭ����ԼΪ6.02��1023 | |
| D�� | ���³�ѹ�£�NA��H2���ӵ����С��22.4L |
| A�� | �ԣ�����H2��=�ԣ��桢HI�� | |
| B�� | �����ڵ���ѹǿ����ʱ����仯 | |
| C�� | ��λʱ��������2n mol HI��ͬʱ������n mol��I2 | |
| D�� | H2��I2��HI�ķ�Ӧ���ʱ�Ϊ2��2��1��״̬ |
������һ��������ܱ������У��������»�ѧ��Ӧ��CO2��g��+H2��g��?CO��g��+H2O��g�����仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���ʾ��
| t/�� | 700 | 800 | 830 | 1 000 | 1 200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=$\frac{c��CO����c��{H}_{2}O��}{c��C{O}_{2}����c��{H}_{2}��}$��
��2���÷�ӦΪ���ȷ�Ӧ������ȡ����ȡ�����
��3�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������BC��
A��������ѹǿ���� B�����������c��CO������ C��v��H2����=v��H2O���� D��c��CO2��=c��CO��
��4����800��ʱ������������Ӧ��ijһʱ�̲�������ڸ����ʵ�Ũ�ȷֱ�Ϊc��CO2��Ϊ2mol/L��c��H2��Ϊ1.5mol/L��c��CO��Ϊ1mol/L��c��H2O��Ϊ3mol/L������һʱ�̣���Ӧ������������������У�
 ��֪X��Y��Z��J��Q���ֶ���������Ԫ�أ�ԭ��������������Ԫ��Z�ڵؿ��к�����ߣ�JԪ�ص���ɫ��Ӧ�ʻ�ɫ��Q�������������������������Ϊ3��8��X����J�γ����ӻ������J+�İ뾶����X-�İ뾶��Y2�ǿ�����Ҫ�ɷ�֮һ����ش�
��֪X��Y��Z��J��Q���ֶ���������Ԫ�أ�ԭ��������������Ԫ��Z�ڵؿ��к�����ߣ�JԪ�ص���ɫ��Ӧ�ʻ�ɫ��Q�������������������������Ϊ3��8��X����J�γ����ӻ������J+�İ뾶����X-�İ뾶��Y2�ǿ�����Ҫ�ɷ�֮һ����ش�