��Ŀ����
5��ҽ������ҩ��������E��ʳƷ������J�ĺϳ�·�����£�
��֪��I��M����E���ӽṹ�е�һ����
II��

��ش��������⣺
��1��A���ڷ���������ṹ��ʽ��
 ��
����2��E�����������ŵ������ǰ�����������
��3��C����NaHCO3��Һ��Ӧ����Ӧ�ٵĻ�ѧ����ʽ��
 ��
����4����Ӧ�ڡ������Լ�ii���Լ�iii���������Ը��������Һ������������Һ��
��5��I��һ�������¿��γɸ߷��ӻ�����仯ѧ����ʽΪ
 ��
����6��J�ж���ͬ���칹�壬���з�������������ͬ���칹����6�֣�
a��Ϊ���Ķ�Ԫȡ�������һ��ȡ����Ϊ�ǻ� b����J������ͬ�Ĺ����ţ����ܷ���������Ӧ
��7����AΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�Ϳ��Һ����Ҫ�ɷ��Ǽ������飨
 ����д���ϳ�·�ߣ��ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������
����д���ϳ�·�ߣ��ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ�������� ��
��
���� �������̣�ͻ�ƿ�ΪF��C7H7Cl�����ȷ�������һ�����̣�AΪ����������Cl2/FeCl3�����·��������ϵ�±������F����AӦΪC7H8������AΪ ��FΪ
��FΪ ��J�к���������Ϊ�Ȼ����ǻ���Ӧ���������������з�Ӧ�������Ȼ������Լ�iiΪ���Ը��������Һ��������ӦΪ�Ȼ�����GΪ
��J�к���������Ϊ�Ȼ����ǻ���Ӧ���������������з�Ӧ�������Ȼ������Լ�iiΪ���Ը��������Һ��������ӦΪ�Ȼ�����GΪ ��J�к��з��ǻ���Ӧ��ClΪ-OHȡ���õ�������iiiΪ����������Һ����HΪ
��J�к��з��ǻ���Ӧ��ClΪ-OHȡ���õ�������iiiΪ����������Һ����HΪ �������ữ���õ�I����IΪ
�������ữ���õ�I����IΪ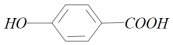 �����Լ�i�м��ȣ�����������Ӧ������J�����Լ�iΪCH3CH2OH���ٷ�������һ�����̣�A��Ũ���ᣬŨ������������£�����������Ӧ���ڶ�λ����BΪ
�����Լ�i�м��ȣ�����������Ӧ������J�����Լ�iΪCH3CH2OH���ٷ�������һ�����̣�A��Ũ���ᣬŨ������������£�����������Ӧ���ڶ�λ����BΪ ���������Ը��������Һ��Ӧ���������ᣬ��CΪ
���������Ը��������Һ��Ӧ���������ᣬ��CΪ �����Լ�i����CH3CH2OH����������Ӧ������D����DΪ
�����Լ�i����CH3CH2OH����������Ӧ������D����DΪ ��������Fe/HCl�����£�����ԭΪ-NH2����EΪ
��������Fe/HCl�����£�����ԭΪ-NH2����EΪ ���ݴ˷������
���ݴ˷������
��� �⣺A�� ��B��
��B�� ��C��
��C�� ��D��
��D�� ��E��
��E�� ��F��
��F�� ��G��
��G�� ��H��
��H�� ��I��
��I�� ���Լ�i��CH3CH2OH���Լ�ii�����Ը��������Һ���Լ�iii��NaOH��Һ��
���Լ�i��CH3CH2OH���Լ�ii�����Ը��������Һ���Լ�iii��NaOH��Һ��
��1��A���ڷ���������ṹ��ʽ�� ��
��
�ʴ�Ϊ�� ��
��
��2��EΪ�� �����к��еĹ�����Ϊ-NH2��-COO-�������Ʒֱ�Ϊ��������������
�����к��еĹ�����Ϊ-NH2��-COO-�������Ʒֱ�Ϊ��������������
�ʴ�Ϊ��������������
��3��CΪ�� ������NaHCO3��Һ��Ӧ����Ӧ��ΪC��CH3CH2OH����������Ӧ������
������NaHCO3��Һ��Ӧ����Ӧ��ΪC��CH3CH2OH����������Ӧ������ ����Ӧ����ʽΪ��
����Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4����Ӧ�ڡ������Լ�ii���Լ�iii���������Ը��������Һ������������Һ��
�ʴ�Ϊ�����Ը��������Һ������������Һ��
��5��IΪ�� ����һ�������¿��γɸ߷��ӻ������Ӧ����ʽΪ��
����һ�������¿��γɸ߷��ӻ������Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��6��JΪ�� ���ж���ͬ���칹�壬���з���a��Ϊ���Ķ�Ԫȡ�������һ��ȡ����Ϊ�ǻ� b����J������ͬ�Ĺ����ţ����ܷ���������Ӧ��ͬ���칹���У�
���ж���ͬ���칹�壬���з���a��Ϊ���Ķ�Ԫȡ�������һ��ȡ����Ϊ�ǻ� b����J������ͬ�Ĺ����ţ����ܷ���������Ӧ��ͬ���칹���У�
������6�֣�
�ʴ�Ϊ��6��
��7��AΪ�� ����AΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�Ϳ��Һ����Ҫ�ɷ��Ǽ������飨
����AΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�Ϳ��Һ����Ҫ�ɷ��Ǽ������飨 �����ɿ����������̣�
�����ɿ����������̣� ��
��
�ʴ�Ϊ�� ��
��
���� ������Ҫ�����л��ϳ����ƶϣ���Ҫ���ճ������л���Ӧ������������ã���ϸ��������ͼ�ǽ��Ĺؼ��������Ѷ��еȣ����е��⣮

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�| A�� | Fe��Cl2��Ӧ����FeCl3����Fe��I2��Ӧ������FeI3 | |
| B�� | Al��OH��3��Cu��OH��2�����ֽ⣬��Fe��OH��3����Ҳ�ֽ� | |
| C�� | Na������ˮ��Ӧ����������KҲ������ˮ��Ӧ�������� | |
| D�� | CO2����ʹ�����ʯ��ˮ����ǣ�SO2Ҳ����ʹ�����ʯ��ˮ����� |
| A�� | ��λʱ��������n mol A��ͬʱ����n mol D | |
| B�� | B��Ũ�ȱ��ֲ��� | |
| C�� | �����ڻ��������ܶȲ���ʱ����仯 | |
| D�� | ��λʱ��������n mol B��ͬʱ����n mol A |
| A�� | Cl2������ | |
| B�� | NH3�õ����� | |
| C�� | Cl2�������� | |
| D�� | ��Ӧÿ����1molN2ת�Ƶ�����ԼΪ8��6.02��1023 |
��
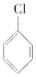 ��
��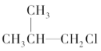 �ۣ�CH3��3C-CH2Cl ��CHCl2-CHBr2 ��
�ۣ�CH3��3C-CH2Cl ��CHCl2-CHBr2 ��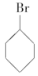 ��CH3Cl��
��CH3Cl��| A�� | �٢ۢ� | B�� | �ڢۢ� | C�� | �ڢ� | D�� | ȫ�� |
| A�� | 1.6g���麬�еĵ�����ΪNA | |
| B�� | 1L0.1mol•L-1NaHCO3��Һ�к��е�HCO3-��ĿΪ0.1NA | |
| C�� | 1LpH=1��������Һ�к��е�H+��Ϊ0.2NA | |
| D�� | ��״���£�2.24LCO��CO2��������к��е���ԭ����Ϊ0.15NA |


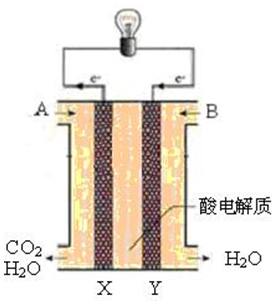

 �о�NOx��SO2��CO�ȴ�����Ⱦ����Ĵ�������������Ҫ���壮
�о�NOx��SO2��CO�ȴ�����Ⱦ����Ĵ�������������Ҫ���壮