��Ŀ����
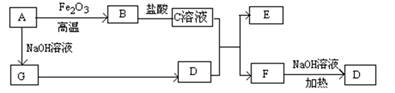
��12�֣���ʽ̼����þ[MgaAlb(OH)c(CO3)d��x H2O]������������ȼ����
��1����ʽ̼����þ������ȼ���ã������������ȷֽ������մ��������� ��
��2��MgaAlb(OH)c(CO3)d��x H2O��a��b��c��d�Ĵ�����ϵʽΪ ��
��3��Ϊȷ����ʽ̼����þ����ɣ���������ʵ�飺
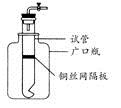
��ȷ��ȡ3.390g��Ʒ������ϡ�����ַ�Ӧ������CO20.560L���ѻ���ɱ�״���£�������ȡһ������Ʒ�ڿ����м��ȣ���Ʒ�Ĺ�������ʣ�������Ʒ��ʣ������/������Ʒ����ʼ������100%�����¶ȵı仯����ͼ��ʾ����Ʒ��2700Cʱ����ȫʧȥ�ᾧˮ��6000C���ϲ�������Ϊ����������Ļ�����
��������ʵ�����ݼ����ʽ̼����þ��Ʒ�е�n(OH��): n(CO32��)��д��������̣���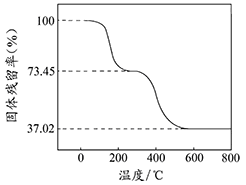
��ֹȼ��
2a+3b=c+2d
��3��
�������������(1�����ȷֽ�Ϊ���۵������þ�������������ڿ�ȼ����棬��ֹȼ�գ���2�������и�Ԫ�صĺϼ۴�����Ϊ0��2a+3b=c+2d����3�����ݷֽ�ͼ�����֪��Ϣ���ó���һ����ʧȥ�ᾧˮ���ڶ����Ƿֽ�Ϊ�����Ӧ���ݺ�һ�μ���OH-��CO32-�Ĺ�ϵ������ʱǰ������ˮ���������ɶ�����̼�����ߵĺͿ��Ը���ͼ�е����ݻ��㣬�ٸ��ݶ�����̼�����������ˮ�������������õ�OH-��CO32-�����ʵ�����
���㣺���⿼�黯ѧ���㡣

�ؾ�ʯ����Ҫ�ɷ�Ϊ���ᱵ����Ϊ����Fe2O3��MnO��̿�ʵ����ʶ��������ɫ����ҵ�Ͻ��ؾ�ʯ�����������ᡢ�����ڷ�Ӧ���л�ϼ��ȣ�����Ư�ס������پ�ˮϴ��һϵ�й����Ƶð�ɫ���ؾ�ʯ���ϣ��㷺����ֽ�š�����ȵ���������֪MnO�Ǽ��������Al�ۿ��Խ���ɫ��Fe3+ת��Ϊdzɫ��Fe2+��
�ؾ�ʯ���ϵ�������������Ϊ��
��1���ڸ������У�Ϊ�ӿ조Ư�ס��ٶȣ���ȡ�Ĵ�ʩ�� ��
��
��2���������ˡ�Ư�ס������á���д������ֱ��������ʱ�Ļ�ѧ����ʽ��
�� ��
��3������Ư��ˮϴ���˺�����������Fe2+���ӵķ�����
�� ��
��4������Ư��ǰ�����յ���ҪĿ���� �������պ�Ĺ���ĥ��ϸ�ۣ�ʹ�õ��Ǽ��и��������ĥ����˵��������кܸߵ� ��
��5����ҵ������Ϊ�˳��������Դ������Һ���������õ�����ԭ��Fe2O3�����������ǣ�
����Һ��ͨ�����Cl2����Ŀ���� ��
���ټ�������NaOH��������Һ��pH ����Ŀ���� ��������Һ��pH��ΧΪ ��
�й����ӿ�ʼ��������ȫ����ʱ��pH���£�
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 7.6 | 9.7 |
| Fe3+ | 2.7 | 3.7 |
| Al3+ | 3.8 | 4.7 |
| Mn2+ | 8.3 | 9.8 |
��6�����ͨ�� �� �õ���������
�ܵ����ʵ���Ϊ0.50 mol��Fe����Al�ۻ�����Ϊ�����������ݣ���һ���м�������
��ϡ���ᣬ�ڱ�״���²�������a L������һ���м�������������������Һ���ڱ�״���²�������b L����a+b����ֵ��������
| A��5.6 | B��7.3 | C��8.2 | D��11.2 |
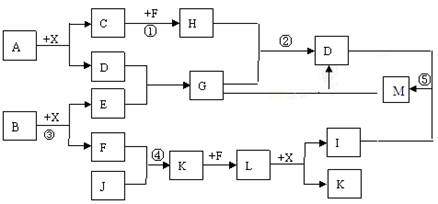
�ú���Al2O3��SiO2������FeO��xFe2O3�������Ʊ�Al2(SO4)3��18H2O��������������(���ֲ�����������)��
��.�������м������ϡH2SO4�����ˣ�
��.����Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
��.���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ��
��.����MnSO4���Ϻ�ɫ��ʧ�����ˣ�
��.Ũ�����ᾧ�����룬�õ���Ʒ��
��1��H2SO4�ܽ�Al2O3�����ӷ���ʽ��____________________________________��
��2����MnO4-����Fe2�������ӷ���ʽ����������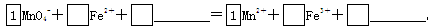
��3����֪��
�����������������pH
| | Al(OH)3 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
���ݱ������ݽ��Ͳ�����Ŀ�ģ�________��
��4����֪��һ�������£�MnO4-����Mn2����Ӧ����MnO2��
�� �� �� �ij����м���ŨHCl�����ȣ���˵�������д���MnO2��������__________��
�ڢ� �м���MnSO4��Ŀ����_____________________________________________��