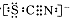��Ŀ����
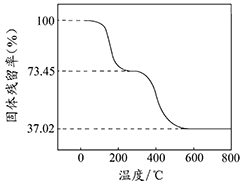
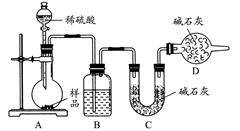
ij��֪A��B���������ֶ�����Ԫ����ɵĻ����A��ijԪ�ص���������Ϊ25%��B����ɫ��Ӧ�ʻ�ɫ��C��J��X��ͬ���ڵ�Ԫ�صļ��⻯�XΪ��ɫҺ�壬C��JΪ���壬D��һ�ֲ�����ˮ�İ�ɫ���塣��Ӧ���ɵ�ˮ������ȥ������������ͼ��ʾ�Ĺ�ϵ��
��1��д����ѧʽ��A_________ E___________ L___________��
��2���ڷ�Ӧ�٢ڢۢܢ�������������ԭ��Ӧ����_____________��
��3����Ӧ�ۻ�ѧ����ʽΪ��______________________________��
��4��д���������ӷ���ʽ����Ӧ�� ��G��Һ��M��Һ�ķ�Ӧ___________��
��1��Al4C3��NaOH��NO2
��2���٢ۢ�
��3��2Na2O2+2H2O��4NaOH+O2��
��4��2AlO2����CO2��3H2O��2Al(OH)3����CO32������AlO2����CO2��2H2O��2Al(OH)3��HCO3������ Al3����3AlO2����6H2O = 4Al(OH)3��
����

��ϰ��ϵ�д�
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
�����Ŀ