��Ŀ����
�ؾ�ʯ����Ҫ�ɷ�Ϊ���ᱵ����Ϊ����Fe2O3��MnO��̿�ʵ����ʶ��������ɫ����ҵ�Ͻ��ؾ�ʯ�����������ᡢ�����ڷ�Ӧ���л�ϼ��ȣ�����Ư�ס������پ�ˮϴ��һϵ�й����Ƶð�ɫ���ؾ�ʯ���ϣ��㷺����ֽ�š�����ȵ���������֪MnO�Ǽ��������Al�ۿ��Խ���ɫ��Fe3+ת��Ϊdzɫ��Fe2+��
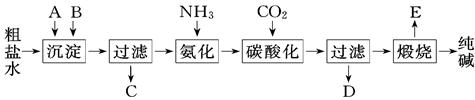
�ؾ�ʯ���ϵ�������������Ϊ��
��1���ڸ������У�Ϊ�ӿ조Ư�ס��ٶȣ���ȡ�Ĵ�ʩ�� ��
��
��2���������ˡ�Ư�ס������á���д������ֱ��������ʱ�Ļ�ѧ����ʽ��
�� ��
��3������Ư��ˮϴ���˺�����������Fe2+���ӵķ�����
�� ��
��4������Ư��ǰ�����յ���ҪĿ���� �������պ�Ĺ���ĥ��ϸ�ۣ�ʹ�õ��Ǽ��и��������ĥ����˵��������кܸߵ� ��
��5����ҵ������Ϊ�˳��������Դ������Һ���������õ�����ԭ��Fe2O3�����������ǣ�
����Һ��ͨ�����Cl2����Ŀ���� ��
���ټ�������NaOH��������Һ��pH ����Ŀ���� ��������Һ��pH��ΧΪ ��
�й����ӿ�ʼ��������ȫ����ʱ��pH���£�
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 7.6 | 9.7 |
| Fe3+ | 2.7 | 3.7 |
| Al3+ | 3.8 | 4.7 |
| Mn2+ | 8.3 | 9.8 |
��6�����ͨ�� �� �õ���������
��1���ؾ�ʯ������ʹ�÷�ĩ��1�֣������ȣ�1�֣���
��2��Fe2O3+3H2SO4�� Fe2(SO4)3+3H2O��1�֣� MnO+H2SO4��MnSO4+H2O��1�֣�
��3��ȡ���һ��ϴ��Һ���Թ��У��ȵμ���ˮ�ٵμ�KSCN��Һ�����Ա仯����1�֣�
��4����ȥ̿�ʣ�1�֣��� Ӳ�ȣ�1�֣���
��5���ٽ�Fe2+ת��ΪFe3+ ��1�֣���ʹFe3+ ������ȫ�����������Ӳ�����������1�֣�3.7��3.8 ��1�֣�
��6�����ˡ�ϴ�ӣ�1�֣������գ�1�֣�
�������������
��1���ؾ�ʯ������ʹ�÷�ĩ���������������ȶ��ɼӿ����ʡ�
��2�����ݿ�ͼ��Ϣ֪��Fe2O3+3H2SO4�� Fe2(SO4)3+3H2O�� MnO+H2SO4��MnSO4+H2O��
��3��������������Fe2+���ӵķ�����ȡ���һ��ϴ��Һ���Թ��У��ȵμ���ˮ�ٵμ�KSCN��Һ�����Ա仯��
��4����������֪����Ϊ��ȥ̿�ʣ������Ӳ�Ⱥܴ�
��5������Һ��ͨ�����Cl2��Fe2+��ȫת��ΪFe3+ ����ʹFe3+ ������ȫ�����������Ӳ�������������ϱ��������ݿ�֪������Һ��pH��Χ3.7��3.8���ɡ�
��6����������Һ�壬�������ˡ�ϴ�ӣ����ռ��ɡ�
���㣺�����Ի�ѧ̽��ʵ��Ϊ������������Ԫ�ؼ����������ʡ�ʵ�������

��ʵ�����ijͬѧȡһС�����������ˮ��Ӧ��ʵ�顣������������⣺
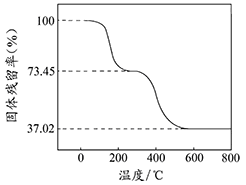
�п��Ľ����Ʊ�¶�ڿ����У����ȹ۲쵽�������� ����������Ӧ�Ļ�ѧ����ʽ�� ��
(2)����Ͷ��ˮ�к����ڻ���һ��С������һ�������ܵó��Ľ����ǣ�
�� ���� ��
��һС����Ͷ��ʢ�б���ʯ��ˮ���ձ��У������ܹ۲쵽�������� �����ţ���
| A������������ | B�����ڻ���С����Һ�����ζ� |
| C����Һ�ײ�������ɫ�Ľ��������� | D����Һ����� |
��4����������ʵ������������������йر仯����˵���������Ʊ�����ú���е�Ŀ���� ��
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã�
�ش��������⣺
| �� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | �� | �� | �� | | | �� | |
��1���ɢ١�������Ԫ����ɵ���Է�������Ϊ28���л���Ŀռ乹���� ��
����������ˮ�����ӳɷ�Ӧ�Ļ�ѧ����ʽ�� ��
��2���õ���ʽ��ʾ�ܵļ��⻯����γɹ������£� ��
��3�� �����ʵ��Ƚ�Ԫ�آ��������Ե����ǿ���� _��
��4�� �â�Ԫ�صĵ������Ԫ�صĵ��ʿ����Ƴɵ�أ������װ��KOHŨ��Һ������Ķ��Ե缫���ҽ���KOH��Һ�У��ڼ�ͨ��ٵĵ��ʣ��Ҽ�ͨ��ܵĵ��ʣ�����ĵ缫��ӦʽΪ�� ��
��5���ɱ��Т١��ۡ��ܡ��ޡ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
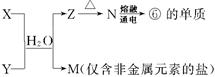
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ ����ҵ�ϳ��õ��ʢ�ұ�����۵Ľ�����д���������͵��ʢ��ڸ����·�Ӧ�Ļ�ѧ����ʽ ��

 NaHCO3��+NH4Cl������ĸҺ�����ַ�����
NaHCO3��+NH4Cl������ĸҺ�����ַ�����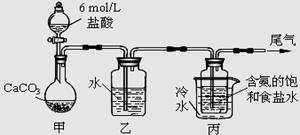

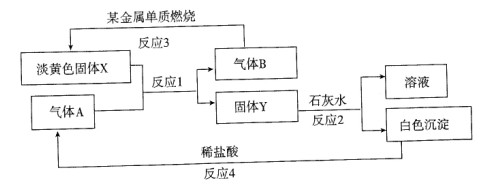
 4AlCl3+3O2 ������ش��������⣺
4AlCl3+3O2 ������ش��������⣺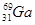 ����ԭ������Ȼͬλ��ԭ������ռ�İٷֱ�Ϊ60%��ʵ�����廯��(GaBr3)��Ħ������Ϊ309.8 g/mol�����ɴ���֪�ص���һ��ͬλ����_________��
����ԭ������Ȼͬλ��ԭ������ռ�İٷֱ�Ϊ60%��ʵ�����廯��(GaBr3)��Ħ������Ϊ309.8 g/mol�����ɴ���֪�ص���һ��ͬλ����_________��